
1-Aminopropan-2-ol is the organic compound with the formula CH3CH(OH)CH2NH2. It is an amino alcohol. The term isopropanolamine may also refer more generally to the additional homologs diisopropanolamine (DIPA) and triisopropanolamine (TIPA).
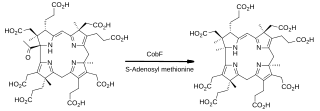
In enzymology, precorrin-6A synthase (deacetylating) (EC 2.1.1.152) is an enzyme that catalyzes the chemical reaction

In enzymology, a precorrin-2 dehydrogenase (EC 1.3.1.76) is an enzyme that catalyzes the chemical reaction
In enzymology, a precorrin-6A reductase (EC 1.3.1.54) is an enzyme that catalyzes the chemical reaction
In enzymology, a precorrin-3B synthase (EC 1.14.13.83) is an enzyme that catalyzes the chemical reaction

In enzymology, a cob(II)yrinic acid a,c-diamide reductase is an enzyme that catalyzes the chemical reaction
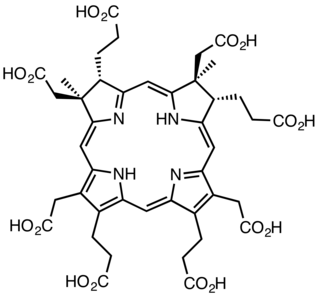
The enzyme sirohydrochlorin cobaltochelatase (EC 4.99.1.3) catalyzes the reaction
The enzyme threonine-phosphate decarboxylase (EC 4.1.1.81) catalyzes the chemical reaction
In enzymology, an adenosylcobyric acid synthase (glutamine-hydrolysing) (EC 6.3.5.10) is an enzyme that catalyzes the chemical reaction

Cobalt chelatase (EC 6.6.1.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a hydrogenobyrinic acid a,c-diamide synthase (glutamine-hydrolysing) (EC 6.3.5.9) is an enzyme that catalyzes the chemical reaction
The primary biochemical reaction catalyzed by the enzyme adenosylcobalamin/α-ribazole phosphatase (formerly α-ribazole phosphatase) (EC 3.1.3.73) is

In enzymology, a nicotinate-nucleotide-dimethylbenzimidazole phosphoribosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an adenosylcobinamide kinase is an enzyme that catalyzes the chemical reaction

In enzymology, an adenosylcobinamide-phosphate guanylyltransferase is an enzyme that catalyzes the chemical reaction
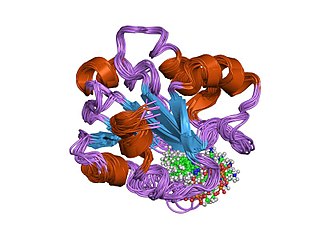
In molecular biology, the vitamin B12-binding domain is a protein domain which binds to cobalamin. It can bind two different forms of the cobalamin cofactor, with cobalt bonded either to a methyl group (methylcobalamin) or to 5'-deoxyadenosine (adenosylcobalamin). Cobalamin-binding domains are mainly found in two families of enzymes present in animals and prokaryotes, which perform distinct kinds of reactions at the cobalt-carbon bond. Enzymes that require methylcobalamin carry out methyl transfer reactions. Enzymes that require adenosylcobalamin catalyse reactions in which the first step is the cleavage of adenosylcobalamin to form cob(II)alamin and the 5'-deoxyadenosyl radical, and thus act as radical generators. In both types of enzymes the B12-binding domain uses a histidine to bind the cobalt atom of cobalamin cofactors. This histidine is embedded in a DXHXXG sequence, the most conserved primary sequence motif of the domain. Proteins containing the cobalamin-binding domain include:

In molecular biology, cob(I)yrinic acid a,c-diamide adenosyltransferase EC 2.5.1.17 is an enzyme which catalyses the conversion of cobalamin into one of its coenzyme forms, adenosylcobalamin. Adenosylcobalamin is required as a cofactor for the activity of certain enzymes. AdoCbl contains an adenosyl moiety liganded to the cobalt ion of cobalamin via a covalent Co-C bond.

Cobalamin biosynthesis is the process by which bacteria and archea make cobalamin, vitamin B12. Many steps are involved in converting aminolevulinic acid via uroporphyrinogen III and adenosylcobyric acid to the final forms in which it is used by enzymes in both the producing organisms and other species, including humans who acquire it through their diet.
5,6-dimethylbenzimidazole synthase (EC 1.14.99.40, BluB) is an enzyme with systematic name FMNH2 oxidoreductase (5,6-dimethylbenzimidazole forming). This enzyme catalyses the following chemical reaction
Adenosylcobinamide-phosphate synthase is an enzyme with systematic name adenosylcobyric acid:(R)-1-aminopropan-2-yl phosphate ligase (ADP-forming). This enzyme catalyses the following chemical reaction











