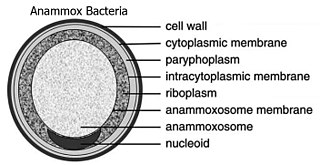
The Planctomycetota are a phylum of widely distributed bacteria, occurring in both aquatic and terrestrial habitats. They play a considerable role in global carbon and nitrogen cycles, with many species of this phylum capable of anaerobic ammonium oxidation, also known as anammox. Many Planctomycetota occur in relatively high abundance as biofilms, often associating with other organisms such as macroalgae and marine sponges.

Anammox, an abbreviation for "anaerobic ammonium oxidation", is a globally important microbial process of the nitrogen cycle that takes place in many natural environments. The bacteria mediating this process were identified in 1999, and were a great surprise for the scientific community. In the anammox reaction, nitrite and ammonium ions are converted directly into diatomic nitrogen and water.
Methanotrophs are prokaryotes that metabolize methane as their source of carbon and chemical energy. They are bacteria or archaea, can grow aerobically or anaerobically, and require single-carbon compounds to survive.
Denitrifying bacteria are a diverse group of bacteria that encompass many different phyla. This group of bacteria, together with denitrifying fungi and archaea, is capable of performing denitrification as part of the nitrogen cycle. Denitrification is performed by a variety of denitrifying bacteria that are widely distributed in soils and sediments and that use oxidized nitrogen compounds such as nitrate and nitrite in the absence of oxygen as a terminal electron acceptor. They metabolize nitrogenous compounds using various enzymes, including nitrate reductase (NAR), nitrite reductase (NIR), nitric oxide reductase (NOR) and nitrous oxide reductase (NOS), turning nitrogen oxides back to nitrogen gas or nitrous oxide.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.

A lithoautotroph is an organism which derives energy from reactions of reduced compounds of mineral (inorganic) origin. Two types of lithoautotrophs are distinguished by their energy source; photolithoautotrophs derive their energy from light while chemolithoautotrophs (chemolithotrophs or chemoautotrophs) derive their energy from chemical reactions. Chemolithoautotrophs are exclusively microbes. Photolithoautotrophs include macroflora such as plants; these do not possess the ability to use mineral sources of reduced compounds for energy. Most chemolithoautotrophs belong to the domain Bacteria, while some belong to the domain Archaea. Lithoautotrophic bacteria can only use inorganic molecules as substrates in their energy-releasing reactions. The term "lithotroph" is from Greek lithos (λίθος) meaning "rock" and trōphos (τροφοσ) meaning "consumer"; literally, it may be read "eaters of rock". The "lithotroph" part of the name refers to the fact that these organisms use inorganic elements/compounds as their electron source, while the "autotroph" part of the name refers to their carbon source being CO2. Many lithoautotrophs are extremophiles, but this is not universally so, and some can be found to be the cause of acid mine drainage.
Nitrifying bacteria are chemolithotrophic organisms that include species of genera such as Nitrosomonas, Nitrosococcus, Nitrobacter, Nitrospina, Nitrospira and Nitrococcus. These bacteria get their energy from the oxidation of inorganic nitrogen compounds. Types include ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB). Many species of nitrifying bacteria have complex internal membrane systems that are the location for key enzymes in nitrification: ammonia monooxygenase, hydroxylamine oxidoreductase, and nitrite oxidoreductase.

In chemistry, a ladderane is an organic molecule containing two or more fused cyclobutane rings. The name arises from the resemblance of a series of fused cyclobutane rings to a ladder. Numerous synthetic approaches have been developed for the synthesis of ladderane compounds of various lengths. The mechanisms often involve [2 + 2] photocycloadditions, a useful reaction for creating strained 4-membered rings. Naturally occurring ladderanes have been identified as major components of the anammoxosome membrane of the anammox bacteria, phylum Planctomycetota.
"CandidatusScalindua brodae" is a bacterial member of the order Planctomycetales and therefore lacks peptidoglycan in its cell wall, has a compartmentalized cytoplasm. It is an ammonium oxidising bacteria.
CandidatusScalindua wagneri is a Gram-negative coccoid-shaped bacterium that was first isolated from a wastewater treatment plant. This bacterium is an obligate anaerobic chemolithotroph that undergoes anaerobic ammonium oxidation (anammox). It can be used in the wastewater treatment industry in nitrogen reactors to remove nitrogenous wastes from wastewater without contributing to fixed nitrogen loss and greenhouse gas emission.
Hydrazine oxidoreductase (EC 1.7.99.8, HAO (ambiguous)) is an enzyme with systematic name hydrazine:acceptor oxidoreductase. This enzyme catalyses the following chemical reaction
Nitrososphaera is a mesophilic genus of ammonia-oxidizing Crenarchaeota. The first Nitrososphaera organism was discovered in garden soils at the University of Vienna leading to the categorization of a new genus, family, order and class of Archaea. This genus is contains three distinct species: N. viennensis, Ca. N. gargensis, and Ca N. evergladensis. Nitrososphaera are chemolithoautotrophs and have important biogeochemical roles as nitrifying organisms.
Candidatus Brocadia fulgida is a prokaryotic species of bacteria that performs the anammox process. Fatty acids constitute an enrichment culture for B. fulgida. The species' 16S ribosomal RNA sequence has been determined. During the anammox process, it oxidizes acetate at the highest rate and outcompetes other anammox bacteria, which indicates that it does not incorporate acetate directly into its biomass like other anammox bacteria.
Dissimilatory nitrate reduction to ammonium (DNRA), also known as nitrate/nitrite ammonification, is the result of anaerobic respiration by chemoorganoheterotrophic microbes using nitrate (NO3−) as an electron acceptor for respiration. In anaerobic conditions microbes which undertake DNRA oxidise organic matter and use nitrate (rather than oxygen) as an electron acceptor, reducing it to nitrite, and then to ammonium (NO3− → NO2− → NH4+).
An oxygen minimum zone (OMZ) is characterized as an oxygen-deficient layer in the world's oceans. Typically found between 200 m to 1500 m deep below regions of high productivity, such as the western coasts of continents. OMZs can be seasonal following the spring-summer upwelling season. Upwelling of nutrient-rich water leads to high productivity and labile organic matter, that is respired by heterotrophs as it sinks down the water column. High respiration rates deplete the oxygen in the water column to concentrations of 2 mg/L or less forming the OMZ. OMZs are expanding, with increasing ocean deoxygenation. Under these oxygen-starved conditions, energy is diverted from higher trophic levels to microbial communities that have evolved to use other biogeochemical species instead of oxygen, these species include nitrate, nitrite, sulphate etc. Several Bacteria and Archea have adapted to live in these environments by using these alternate chemical species and thrive. The most abundant phyla in OMZs are Pseudomonadota, Bacteroidota, Actinomycetota, and Planctomycetota.

NC10 is a bacterial phylum with candidate status, meaning its members remain uncultured to date. The difficulty in producing lab cultures may be linked to low growth rates and other limiting growth factors.
CandidatusAnammoxoglobus propionicus is an anammox bacteria that is taxonomically in the phylum of Planctomycetota. Anammoxoglobus propionicus is an interest to many researchers due to its ability to reduce nitrite and oxidize ammonium into nitrogen gas and water.

Candidatus "Methylomirabilis oxyfera" is a candidate species of Gram-negative bacteria belonging to the NC10 phylum, characterized for its capacity to couple anaerobic methane oxidation with nitrite reduction in anoxic environments. To acquire oxygen for methane oxidation, M. oxyfera utilizes an intra-aerobic pathway through the reduction of nitrite (NO2) to dinitrogen (N2) and oxygen.
"Candidatus Brocadia" is a candidatus genus of bacteria, meaning that while it is well-characterized, it has not been grown as a pure culture yet. Due to this, much of what is known about Candidatus species has been discovered using culture-independent techniques such as metagenomic sequence analysis.
Anammox is a wastewater treatment technique that removes nitrogen using anaerobic ammonium oxidation (anammox). This process is performed by anammox bacteria which are autotrophic, meaning they do not need organic carbon for their metabolism to function. Instead, the metabolism of anammox bacteria convert ammonium and nitrite into dinitrogen gas. Anammox bacteria are a wastewater treatment technique and wastewater treatment facilities are in the process of implementing anammox-based technologies to further enhance ammonia and nitrogen removal.






