
Glycolysis is the metabolic pathway that converts glucose into pyruvate, and in most organisms, occurs in the liquid part of cells, the cytosol. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.
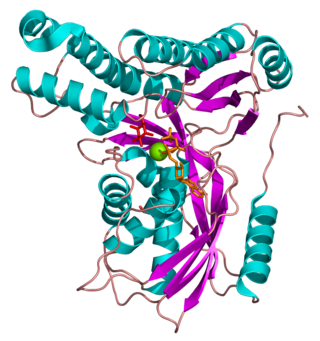
Galactokinase is an enzyme (phosphotransferase) that facilitates the phosphorylation of α-D-galactose to galactose 1-phosphate at the expense of one molecule of ATP. Galactokinase catalyzes the second step of the Leloir pathway, a metabolic pathway found in most organisms for the catabolism of α-D-galactose to glucose 1-phosphate. First isolated from mammalian liver, galactokinase has been studied extensively in yeast, archaea, plants, and humans.

The Entner–Doudoroff pathway is a metabolic pathway that is most notable in Gram-negative bacteria, certain Gram-positive bacteria and archaea. Glucose is the substrate in the ED pathway and through a series of enzyme assisted chemical reactions it is catabolized into pyruvate. Entner and Doudoroff (1952) and MacGee and Doudoroff (1954) first reported the ED pathway in the bacterium Pseudomonas saccharophila. While originally thought to be just an alternative to glycolysis (EMP) and the pentose phosphate pathway (PPP), some studies now suggest that the original role of the EMP may have originally been about anabolism and repurposed over time to catabolism, meaning the ED pathway may be the older pathway. Recent studies have also shown the prevalence of the ED pathway may be more widespread than first predicted with evidence supporting the presence of the pathway in cyanobacteria, ferns, algae, mosses, and plants. Specifically, there is direct evidence that Hordeum vulgare uses the Entner–Doudoroff pathway.

Nicotinamide adenine dinucleotide phosphate, abbreviated NADP+ or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). NADPH is the reduced form of NADP+, the oxidized form. NADP+ is used by all forms of cellular life.

3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P). This glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin-Benson cycle. The anion is often termed as PGA when referring to the Calvin-Benson cycle. In the Calvin-Benson cycle, 3-phosphoglycerate is typically the product of the spontaneous scission of an unstable 6-carbon intermediate formed upon CO2 fixation. Thus, two equivalents of 3-phosphoglycerate are produced for each molecule of CO2 that is fixed. In glycolysis, 3-phosphoglycerate is an intermediate following the dephosphorylation (reduction) of 1,3-bisphosphoglycerate.

Purine nucleoside phosphorylase, PNP, PNPase or inosine phosphorylase is an enzyme that in humans is encoded by the NP gene. It catalyzes the chemical reaction

Phosphoglycerate kinase is an enzyme that catalyzes the reversible transfer of a phosphate group from 1,3-bisphosphoglycerate (1,3-BPG) to ADP producing 3-phosphoglycerate (3-PG) and ATP :
Glucose-1,6-bisphosphate synthase is a type of enzyme called a phosphotransferase and is involved in mammalian starch and sucrose metabolism. It catalyzes the transfer of a phosphate group from 1,3-bisphosphoglycerate to glucose-1-phosphate, yielding 3-phosphoglycerate and glucose-1,6-bisphosphate.

In enzymology, a glycerate dehydrogenase (EC 1.1.1.29) is an enzyme that catalyzes the chemical reaction
In enzymology, a mannosyl-3-phosphoglycerate synthase is an enzyme that catalyzes the chemical reaction
In enzymology, a carbamate kinase (EC 2.7.2.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a glycerate kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a lombricine kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a protein-histidine pros-kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a protein-histidine tele-kinase is an enzyme that catalyzes the chemical reaction

In enzymology, a ribokinase is an enzyme that catalyzes the chemical reaction

In enzymology, a tau-protein kinase is an enzyme that catalyzes the chemical reaction
Glucosyl-3-phosphoglycerate phosphatase (EC 3.1.3.85, GpgP protein) is an enzyme with systematic name α-D-glucosyl-3-phospho-D-glycerate phosphohydrolase. This enzyme catalyses the following chemical reaction
Picrophilus torridus is a species of Archaea described in 1996. Picrophilus torridus was found in soil near a hot spring in Hokkaido, Japan. The pH of the soil was less than 0.5. P. torridus also has one of the smallest genomes found among organisms that are free-living and are non-parasitic and a high coding density, meaning that the majority of its genes are coding regions and provide instructions for building proteins. The current research suggests the two hostile conditions favored by P. torridus have exerted selective pressure towards having a small and compact genome, which is less likely to be damaged by the harsh environment.
Acidilobus saccharovorans is a thermoacidophilic species of anaerobic archaea. The species was originally described in 2009 after being isolated from hot springs in Kamchatka.










