Related Research Articles
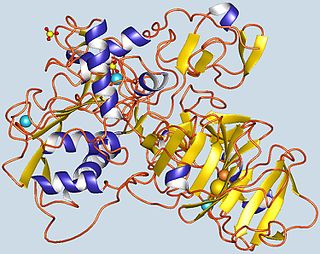
Gelatinase A, also known as MMP2 is an enzyme. This enzyme catalyses the following chemical reaction
Neutrophil collagenase is an enzyme. This enzyme catalyses the following chemical reaction
Microbial collagenase is an enzyme. This enzyme catalyses the following chemical reaction
Clostripain is a proteinase that cleaves proteins on the carboxyl peptide bond of arginine. It was isolated from Clostridium histolyticum. The isoelectric point of the enzyme is 4.8-4.9, and optimum pH is 7.4~7.8. The composition of the enzyme is indicated to be of two chains of relative molecular mass 45,000 and 12,500.
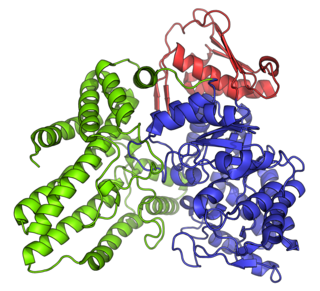
The enzyme α-N-acetylglucosaminidase is a protein associated with Sanfilippo syndrome, with systematic name α-N-acetyl-D-glucosaminide N-acetylglucosaminohydrolase. It catalyses the hydrolysis of terminal non-reducing N-acetyl-D-glucosamine residues in N-acetyl-α-D-glucosaminides, and also UDP-N-acetylglucosamine.
Spermine synthase is an enzyme that converts spermidine into spermine. This enzyme catalyses the following chemical reaction

Neutrophil collagenase, also known as matrix metalloproteinase-8 (MMP-8) or PMNL collagenase (MNL-CL), is a collagen cleaving enzyme which is present in the connective tissue of most mammals. In humans, the MMP-8 protein is encoded by the MMP8 gene. The gene is part of a cluster of MMP genes which localize to chromosome 11q22.3. Most MMP's are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. However, the enzyme encoded by this gene is stored in secondary granules within neutrophils and is activated by autolytic cleavage.
Catalase-peroxidase (EC 1.11.1.21, katG (gene)) is an enzyme with systematic name donor:hydrogen-peroxide oxidoreductase. This enzyme catalyses the following chemical reaction
- donor + H2O2 ⇌ oxidized donor + 2 H2O
- 2 H2O2 ⇌ O2 + 2 H2O
(Methyl-Co methanol-specific corrinoid protein):coenzyme M methyltransferase is an enzyme with systematic name methylated methanol-specific corrinoid protein:coenzyme M methyltransferase. This enzyme catalyses the following chemical reaction
Ribonuclease IV is an enzyme. This enzyme catalyses the following chemical reaction
Aminopeptidase S is an enzyme. This enzyme catalyses the following chemical reaction
Membrane Pro-Xaa carboxypeptidase is an enzyme. This enzyme catalyses the following chemical reaction
Carboxypeptidase T is an enzyme. This enzyme catalyses the following chemical reaction:
Pyroglutamyl-peptidase II is an enzyme. This enzyme catalyses the following chemical reaction
Gamma-D-glutamyl-meso-diaminopimelate peptidase is an enzyme. This enzyme catalyses the following chemical reaction
Endothiapepsin is an enzyme. This enzyme catalyses the following chemical reaction
Procollagen C-endopeptidase is an enzyme. This enzyme catalyses the following chemical reaction
Biotin-independent malonate decarboxylase (EC 4.1.1.88, malonate decarboxylase (without biotin), malonate decarboxylase, MDC) is an enzyme with systematic name malonate carboxy-lyase (biotin-independent). This enzyme catalyses the following chemical reaction
Biotin-dependent malonate decarboxylase (EC 4.1.1.89, malonate decarboxylase (with biotin), malonate decarboxylase) is an enzyme with systematic name malonate carboxy-lyase (biotin-dependent). This enzyme catalyses the following chemical reaction
Acetate—[acyl-carrier protein] ligase is an enzyme with systematic name acetate:(acyl-carrier-protein) ligase (AMP-forming). This enzyme catalyses the following chemical reaction
References
- ↑ Lecroisey A, Boulard C, Keil B (November 1979). "Chemical and enzymatic characterization of the collagenase from the insect Hypoderma lineatum". European Journal of Biochemistry. 101 (2): 385–93. doi: 10.1111/j.1432-1033.1979.tb19730.x . PMID 230030.
- ↑ Lecroisey A, Keil B (October 1985). "Specificity of the collagenase from the insect Hypoderma lineatum". European Journal of Biochemistry. 152 (1): 123–30. doi: 10.1111/j.1432-1033.1985.tb09171.x . PMID 2995028.
- ↑ Lecroisey A, Gilles AM, De Wolf A, Keil B (June 1987). "Complete amino acid sequence of the collagenase from the insect Hypoderma lineatum". The Journal of Biological Chemistry. 262 (16): 7546–51. PMID 3034899.