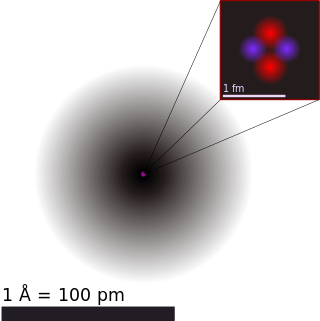
Atomic theory is the scientific theory that matter is composed of particles called atoms. The definition of the word "atom" has changed over the years in response to scientific discoveries. Initially, it referred to a hypothetical concept of there being some fundamental particle of matter, too small to be seen by the naked eye, that could not be divided. Then the definition was refined to being the basic particles of the chemical elements, when chemists observed that elements seemed to combine with each other in ratios of small whole numbers. Then physicists discovered that these particles had an internal structure of their own and therefore perhaps did not deserve to be called "atoms", but renaming atoms would have been impractical by that point.
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during reactions with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds.
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8, meaning each oxygen atom has 8 protons in its nucleus. Atoms of the same element can have different numbers of neutrons in their nuclei, known as isotopes of the element. Two or more atoms can combine to form molecules. Some elements are formed from molecules of identical atoms, e. g. atoms of hydrogen (H) form diatomic molecules (H2). Chemical compounds are substances made of atoms of different elements; they can have molecular or non-molecular structure. Mixtures are materials containing different chemical substances; that means (in case of molecular substances) that they contain different types of molecules. Atoms of one element can be transformed into atoms of a different element in nuclear reactions, which change an atom's atomic number.
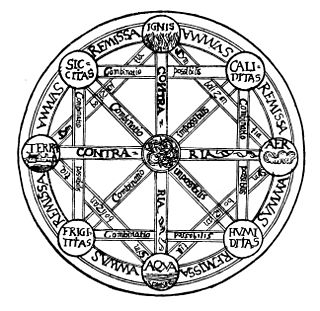
The classical elements typically refer to earth, water, air, fire, and (later) aether which were proposed to explain the nature and complexity of all matter in terms of simpler substances. Ancient cultures in Greece, Angola, Tibet, India, and Mali had similar lists which sometimes referred, in local languages, to "air" as "wind", and to "aether" as "space".
Earth is one of the classical elements, in some systems being one of the four along with air, fire, and water.

Wuxing, usually translated as Five Phases or Five Agents, is a fivefold conceptual scheme used in many traditional Chinese fields of study to explain a wide array of phenomena, including cosmic cycles, the interactions between internal organs, the succession of political regimes, and the properties of herbal medicines.

The phlogiston theory, a superseded scientific theory, postulated the existence of a fire-like element dubbed phlogiston contained within combustible bodies and released during combustion. The name comes from the Ancient Greek φλογιστόνphlogistón, from φλόξphlóx (flame). The idea of a phlogistic substance was first proposed in 1667 by Johann Joachim Becher and later put together more formally in 1703 by Georg Ernst Stahl. Phlogiston theory attempted to explain chemical processes such as combustion and rusting, now collectively known as oxidation. The theory was challenged by the concomitant weight increase and was abandoned before the end of the 18th century following experiments by Antoine Lavoisier in the 1770s and by other scientists. Phlogiston theory led to experiments that ultimately resulted in the identification, and naming (1777), of oxygen by Joseph Priestley and Antoine Lavoisier, respectively.

Chemical symbols are the abbreviations used in chemistry, mainly for chemical elements; but also for functional groups, chemical compounds, and other entities. Element symbols for chemical elements, also known as atomic symbols, normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised.
A period 5 element is one of the chemical elements in the fifth row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour begins to repeat, meaning that elements with similar behaviour fall into the same vertical columns. The fifth period contains 18 elements, beginning with rubidium and ending with xenon. As a rule, period 5 elements fill their 5s shells first, then their 4d, and 5p shells, in that order; however, there are exceptions, such as rhodium.
A period 2 element is one of the chemical elements in the second row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.

The periodic table is an arrangement of the chemical elements, structured by their atomic number, electron configuration and recurring chemical properties. In the basic form, elements are presented in order of increasing atomic number, in the reading sequence. Then, rows and columns are created by starting new rows and inserting blank cells, so that rows (periods) and columns (groups) show elements with recurring properties. For example, all elements in group (column) 18 are noble gases that are largely—though not completely—unreactive.

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis of the various branches of chemistry. Examples include the discovery of fire, extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.

Meteorology is a treatise by Aristotle. The text discusses what Aristotle believed to have been all the affections common to air and water, and the kinds and parts of the Earth and the affections of its parts. It includes early accounts of water evaporation, earthquakes, and other weather phenomena.
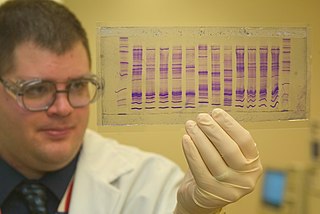
Forensic chemistry is the application of chemistry and its subfield, forensic toxicology, in a legal setting. A forensic chemist can assist in the identification of unknown materials found at a crime scene. Specialists in this field have a wide array of methods and instruments to help identify unknown substances. These include high-performance liquid chromatography, gas chromatography-mass spectrometry, atomic absorption spectroscopy, Fourier transform infrared spectroscopy, and thin layer chromatography. The range of different methods is important due to the destructive nature of some instruments and the number of possible unknown substances that can be found at a scene. Forensic chemists prefer using nondestructive methods first, to preserve evidence and to determine which destructive methods will produce the best results.
Atomism is a natural philosophy proposing that the physical universe is composed of fundamental indivisible components known as atoms.

A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combined without reacting, they may form a chemical mixture. If a mixture is separated to isolate one chemical substance to a desired degree, the resulting substance is said to be chemically pure.
Aristotelian physics is the form of natural philosophy described in the works of the Greek philosopher Aristotle. In his work Physics, Aristotle intended to establish general principles of change that govern all natural bodies, both living and inanimate, celestial and terrestrial – including all motion, quantitative change, qualitative change, and substantial change. To Aristotle, 'physics' was a broad field including subjects which would now be called the philosophy of mind, sensory experience, memory, anatomy and biology. It constitutes the foundation of the thought underlying many of his works.
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment. Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features an extensive vocabulary and a significant amount of jargon.
Chemistry: A Volatile History is a 2010 BBC documentary on the history of chemistry presented by Jim Al-Khalili. It was nominated for the 2010 British Academy Television Awards in the category Specialist Factual.









