
Metal cluster compounds are a molecular ion or neutral compound composed of three or more metals and featuring significant metal-metal interactions. [2]

Metal cluster compounds are a molecular ion or neutral compound composed of three or more metals and featuring significant metal-metal interactions. [2]
The development of metal carbonyl clusters such as Ni(CO)4 and Fe(CO)5 led quickly to the isolation of Fe2(CO)9 and Fe3(CO)12. Rundle and Dahl discovered that Mn2(CO)10 featured an "unsupported" Mn-Mn bond, thereby verifying the ability of metals to bond to one another in molecules. In the 1970s, Paolo Chini demonstrated that very large clusters could be prepared from the platinum metals, one example being [Rh13(CO)24H3]2−. This area of cluster chemistry has benefited from single-crystal X-ray diffraction.

Many metal carbonyl clusters contain ligands aside from CO. For example, the CO ligand can be replaced with myriad alternatives such as phosphines, isocyanides, alkenes, hydride, etc. Some carbonyl clusters contain two or more metals. Others contain carbon vertices. One example is the methylidyne-tricobalt cluster [Co3(CH)(CO)9]. [3] The above-mentioned cluster serves as an example of an overall zero-charged (neutral) cluster. In addition, cationic (positively charged) rather than neutral organometallic trimolybdenum [4] [5] or tritungsten [6] clusters are also known. The first representative of these ionic organometallic clusters is [Mo3(CCH3)2(O2CCH3)6(H2O)3]2+.

The halides of low-valent early metals often are clusters with extensive M-M bonding. The situation contrasts with the higher halides of these metals and virtually all halides of the late transition metals, where metal-halide bonding is replete.
Transition metal halide clusters are prevalent for the heavier metals: Zr, Hf, Nb, Ta, Mo, W, and Re. For the earliest metals Zr and Hf, interstitial carbide ligands are also common. One example is Zr6CCl12. [7] One structure type features six terminal halides and 12 edge-bridging halides. This motif is exemplified by tungsten(III) chloride, [Ta6Cl18]4−, [8] Another common structure has six terminal halides and 8 bridging halides, e.g. Mo6Cl142−.
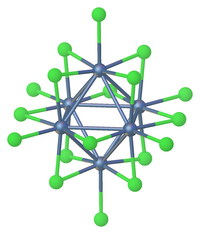
Many of the early metal clusters can only be prepared when they incorporate interstitial atoms.
In terms of history, Linus Pauling showed that "MoCl2" consisted of Mo6 octahedra. F. Albert Cotton established that "ReCl3" in fact features subunits of the cluster Re3Cl9, which could be converted to a host of adducts without breaking the Re-Re bonds. Because this compound is diamagnetic and not paramagnetic the rhenium bonds are double bonds and not single bonds. In the solid state further bridging occurs between neighbours and when this compound is dissolved in hydrochloric acid a Re3Cl123− complex forms. An example of a tetranuclear complex is hexadecamethoxytetratungsten W4(OCH3)12 with tungsten single bonds. A related group of clusters with the general formula MxMo6X8 such as PbMo6S8. These sulfido clusters are called Chevrel phases.
In the 1970s, ferredoxin was demonstrated to contain Fe4S4 clusters and later nitrogenase was shown to contain a distinctive MoFe7S9 active site. [10] The Fe-S clusters mainly serve as redox cofactors, but some have a catalytic function. In the area of bioinorganic chemistry, a variety of Fe-S clusters have also been identified that have CO as ligands.
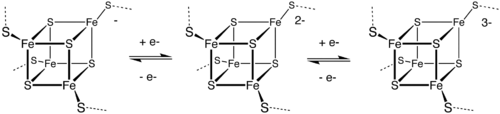

FeMoco, the active site of most nitrogenases, features a Fe7MoS9C cluster. [11]
Zintl compounds feature naked anionic clusters that are generated by reduction of heavy main group p elements, mostly metals or semimetals, with alkali metals, often as a solution in anhydrous liquid ammonia or ethylenediamine. [12] Examples of Zintl anions are [Bi3]3−, [Sn9]4−, [Pb9]4−, and [Sb7]3−. [13] Although these species are called "naked clusters," they are usually strongly associated with alkali metal cations. Some examples have been isolated using cryptate complexes of the alkali metal cation, e.g., [Pb10]2− anion, which features a capped square antiprismatic shape. [14] According to Wade's rules (2n+2) the number of cluster electrons is 22 and therefore a closo cluster. The compound is prepared from oxidation of K4Pb9 [15] by Au+ in PPh3AuCl (by reaction of tetrachloroauric acid and triphenylphosphine) in ethylene diamine with 2.2.2-crypt. This type of cluster was already known as is the endohedral Ni@Pb102− (the cage contains one nickel atom). The icosahedral tin cluster Sn122− or stannaspherene anion is another closed shell structure observed (but not isolated) with photoelectron spectroscopy. [16] [17] With an internal diameter of 6.1 Ångstrom, it is of comparable size to fullerene and should be capable of containing small atoms in the same manner as endohedral fullerenes, and indeed exists a Sn12 cluster that contains an Ir atom: [Ir@Sn12]3−. [18]
Elementoid clusters are ligand-stabilized clusters of metal compounds that possess more direct element-element than element-ligand contacts. Examples of structurally characterized clusters feature ligand stabilized cores of Al77, Ga84, and Pd145. [19]
These clusters consist of at least two different (semi)metallic elements, and possess more direct metal-metal than metal-ligand contacts. The suffix "oid" designate that such clusters possess at a molecular scale, atom arrangements that appear in bulk intermetallic compounds with high coordination numbers of the atoms, such as for example in Laves phase and Hume-Rothery phases. [20] Ligand-free intermetalloid clusters include also endohedrally filled Zintl clusters. [13] [21] A synonym for ligand-stabilized intermetalloid clusters is "molecular alloy". The clusters appear as discrete units in intermetallic compounds separated from each other by electropositive atoms such as [Sn@Cu12@Sn20]12−, [20] as soluble ions [As@Ni12@As20]3− [13] or as ligand-stabilized molecules such as [Mo(ZnCH3)9(ZnCp*)3]. [22]

A metallocene is a compound typically consisting of two cyclopentadienyl anions (C
5H−
5, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula (C5H5)2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride or vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze olefin polymerization.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.
In organometallic chemistry, acetylide refers to chemical compounds with the chemical formulas MC≡CH and MC≡CM, where M is a metal. The term is used loosely and can refer to substituted acetylides having the general structure RC≡CM. Acetylides are reagents in organic synthesis. The calcium acetylide commonly called calcium carbide is a major compound of commerce.
In chemistry, a superatom is any cluster of atoms that seem to exhibit some of the properties of elemental atoms.
A transition metal carbene complex is an organometallic compound featuring a divalent carbon ligand, itself also called a carbene. Carbene complexes have been synthesized from most transition metals and f-block metals, using many different synthetic routes such as nucleophilic addition and alpha-hydrogen abstraction. The term carbene ligand is a formalism since many are not directly derived from carbenes and most are much less reactive than lone carbenes. Described often as =CR2, carbene ligands are intermediate between alkyls (−CR3) and carbynes (≡CR). Many different carbene-based reagents such as Tebbe's reagent are used in synthesis. They also feature in catalytic reactions, especially alkene metathesis, and are of value in both industrial heterogeneous and in homogeneous catalysis for laboratory- and industrial-scale preparation of fine chemicals.
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process. A palladium (0) species is generally utilized as the catalyst, though nickel is sometimes used. A variety of nickel catalysts in either Ni0 or NiII oxidation state can be employed in Negishi cross couplings such as Ni(PPh3)4, Ni(acac)2, Ni(COD)2 etc.
Iron shows the characteristic chemical properties of the transition metals, namely the ability to form variable oxidation states differing by steps of one and a very large coordination and organometallic chemistry: indeed, it was the discovery of an iron compound, ferrocene, that revolutionalized the latter field in the 1950s. Iron is sometimes considered as a prototype for the entire block of transition metals, due to its abundance and the immense role it has played in the technological progress of humanity. Its 26 electrons are arranged in the configuration [Ar]3d64s2, of which the 3d and 4s electrons are relatively close in energy, and thus it can lose a variable number of electrons and there is no clear point where further ionization becomes unprofitable.

Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.

In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives. Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.
In chemistry, a Zintl phase is a product of a reaction between a group 1 or group 2 and main group metal or metalloid. It is characterized by intermediate metallic/ionic bonding. Zintl phases are a subgroup of brittle, high-melting intermetallic compounds that are diamagnetic or exhibit temperature-independent paramagnetism and are poor conductors or semiconductors.

Group 2 organometallic chemistry refers to the chemistry of compounds containing carbon bonded to any group 2 element. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organometallic group 2 compounds are rare and are typically limited to academic interests.
A stannide can refer to an intermetallic compound containing tin combined with one or more other metals; an anion consisting solely of tin atoms or a compound containing such an anion, or, in the field of organometallic chemistry an ionic compound containing an organotin anion
Organogold chemistry is the study of compounds containing gold–carbon bonds. They are studied in academic research, but have not received widespread use otherwise. The dominant oxidation states for organogold compounds are I with coordination number 2 and a linear molecular geometry and III with CN = 4 and a square planar molecular geometry.

Transition metal alkyl complexes are coordination complexes that contain a bond between a transition metal and an alkyl ligand. Such complexes are not only pervasive but are of practical and theoretical interest.
The phosphaethynolate anion, also referred to as PCO, is the phosphorus-containing analogue of the cyanate anion with the chemical formula [PCO]− or [OCP]−. The anion has a linear geometry and is commonly isolated as a salt. When used as a ligand, the phosphaethynolate anion is ambidentate in nature meaning it forms complexes by coordinating via either the phosphorus or oxygen atoms. This versatile character of the anion has allowed it to be incorporated into many transition metal and actinide complexes but now the focus of the research around phosphaethynolate has turned to utilising the anion as a synthetic building block to organophosphanes.
The telluride bromides are chemical compounds that contain both telluride ions (Te2−) and bromide ions (Br−). They are in the class of mixed anion compounds or chalcogenide halides.
Gallium monoiodide is an inorganic gallium compound with the formula GaI or Ga4I4. It is a pale green solid and mixed valent gallium compound, which can contain gallium in the 0, +1, +2, and +3 oxidation states. It is used as a pathway for many gallium-based products. Unlike the gallium(I) halides first crystallographically characterized, gallium monoiodide has a more facile synthesis allowing a synthetic route to many low-valent gallium compounds.
An arsinide, arsanide, dihydridoarsenate(1−) or arsanyl compound is a chemical derivative of arsine, where one hydrogen atom is replaced with a metal or cation. The arsinide ion has formula AsH−2. It can be considered as a ligand with name arsenido or arsanido. Few chemists study arsanyl compounds, as they are both toxic and unstable. The IUPAC names are arsanide and dihydridoarsenate(1−). For the ligand the name is arsanido. The neutral −AsH2 group is termed arsanyl.

Organoberyllium chemistry involves the synthesis and properties of organometallic compounds featuring the group 2 alkaline earth metal beryllium (Be). The area remains understudied, relative to the chemistry of other main-group elements, because although metallic beryllium is relatively unreactive, its dust causes berylliosis and compounds are toxic. Organoberyllium compounds are typically prepared by transmetallation or alkylation of beryllium chloride.
Thallides are compounds containing anions composed of thallium. There are several thallium atoms in a cluster, and it does not occur as a single Tl− in thallides. They are a subclass of trielides, which also includes gallides and indides. A more general classification is polar intermetallics, as clusters contain delocalized multicentre bonds. Thallides were discovered by Eduard Zintl in 1932.