
Glass is a non-crystalline, often transparent amorphous solid, that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenching) of the molten form; some glasses such as volcanic glass are naturally occurring. The most familiar, and historically the oldest, types of manufactured glass are "silicate glasses" based on the chemical compound silica, the primary constituent of sand. Soda-lime glass, containing around 70% silica, accounts for around 90% of manufactured glass. The term glass, in popular usage, is often used to refer only to this type of material, although silica-free glasses often have desirable properties for applications in modern communications technology. Some objects, such as drinking glasses and eyeglasses, are so commonly made of silicate-based glass that they are simply called by the name of the material.

Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as synthetic product. Notable examples include fused quartz, fumed silica, silica gel, and aerogels. It is used in structural materials, microelectronics (as an electrical insulator), and as components in the food and pharmaceutical industries.

Slag is the glass-like by-product left over after a desired metal has been separated from its raw ore. Slag is usually a mixture of metal oxides and silicon dioxide. However, slags can contain metal sulfides and elemental metals. While slags are generally used to remove waste in metal smelting, they can also serve other purposes, such as assisting in the temperature control of the smelting, and minimizing any re-oxidation of the final liquid metal product before the molten metal is removed from the furnace and used to make solid metal. In some smelting processes, such as ilmenite smelting to produce titanium dioxide, the slag is the valuable product instead of the metal.

In the field of optics, transparency is the physical property of allowing light to pass through the material without appreciable scattering of light. On a macroscopic scale, the photons can be said to follow Snell's Law. Translucency allows light to pass through, but does not necessarily follow Snell's law; the photons can be scattered at either of the two interfaces, or internally, where there is a change in index of refraction. In other words, a translucent material is made up of components with different indices of refraction. A transparent material is made up of components with a uniform index of refraction. Transparent materials appear clear, with the overall appearance of one color, or any combination leading up to a brilliant spectrum of every color. The opposite property of translucency is opacity.
A silver halide is one of the chemical compounds that can form between the element silver and one of the halogens. In particular, bromine, chlorine, iodine and fluorine may each combine with silver to produce silver bromide (AgBr), silver chloride (AgCl), silver iodide (AgI), and three forms of silver fluoride, respectively.

Tellurium dioxide (TeO2) is a solid oxide of tellurium. It is encountered in two different forms, the yellow orthorhombic mineral tellurite, β-TeO2, and the synthetic, colourless tetragonal (paratellurite), α-TeO2. Most of the information regarding reaction chemistry has been obtained in studies involving paratellurite, α-TeO2.
ZBLAN is the most stable, and consequently the most used, fluoride glass, a subcategory of the heavy metal fluoride glass (HMFG) group. Typically its composition is 53% ZrF4, 20% BaF2, 4% LaF3, 3% AlF3 and 20% NaF. ZBLAN is not a single material but rather has a spectrum of compositions, many of which are still untried. The biggest library in the world of ZBLAN glass compositions is currently owned by Le Verre Fluore, the oldest company working on HMFG technology. Hafnium fluoride is chemically similar to zirconium fluoride, and is sometimes used in place of it.

An optical fiber is a flexible, transparent fiber made by drawing glass (silica) or plastic to a diameter slightly thicker than that of a human hair. Optical fibers are used most often as a means to transmit light between the two ends of the fiber and find wide usage in fiber-optic communications, where they permit transmission over longer distances and at higher bandwidths than electrical cables. Fibers are used instead of metal wires because signals travel along them with less loss; in addition, fibers are immune to electromagnetic interference, a problem from which metal wires suffer. Fibers are also used for illumination and imaging, and are often wrapped in bundles so they may be used to carry light into, or images out of confined spaces, as in the case of a fiberscope. Specially designed fibers are also used for a variety of other applications, some of them being fiber optic sensors and fiber lasers.
Germanium dioxide, also called germanium oxide, germania, and salt of germanium, is an inorganic compound with the chemical formula GeO2. It is the main commercial source of germanium. It also forms as a passivation layer on pure germanium in contact with atmospheric oxygen.

Fluoride glass is a class of non-oxide optical glasses composed of fluorides of various metals. Due to their low viscosity, it is very difficult to completely avoid the occurrence of any crystallization while processing it through the glass transition.
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification.
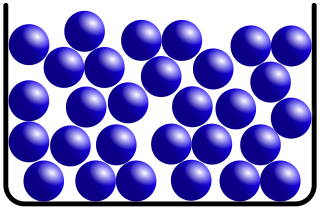
The structure of liquids, glasses and other non-crystalline solids is characterized by the absence of long-range order which defines crystalline materials. Liquids and amorphous solids do, however, possess a rich and varied array of short to medium range order, which originates from chemical bonding and related interactions. Metallic glasses, for example, are typically well described by the dense random packing of hard spheres, whereas covalent systems, such as silicate glasses, have sparsely packed, strongly bound, tetrahedral network structures. These very different structures result in materials with very different physical properties and applications.
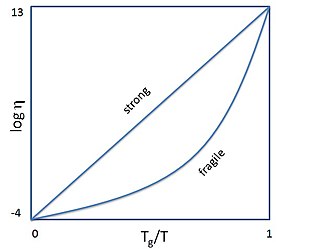
In glass physics, fragility characterizes how rapidly the dynamics of a material slow down as it is cooled toward the glass transition: materials with a higher fragility have a relatively narrow glass transition temperature range, while those with low fragility have a relatively broad glass transition temperature range. Physically, fragility may be related to the presence of dynamical heterogeneity in glasses, as well as to the breakdown of the usual Stokes–Einstein relationship between viscosity and diffusion.
Barium metaphosphate is an inorganic substance with the molecular formula Ba(PO3)2. It is a colourless solid that is insoluble in water, though is soluble in acidic solutions through "slow dissolution". X-ray crystallography shows that this material is composed of Ba2+ cations attached to a polyphosphate ((PO3−)n) anion. A number of hydrated forms are known which are actually cyclic metaphosphates, Ba2(P4O12)·3.5H2O, Ba3(P3O9)2·6H2O.
The glass forming ability of gallium(III) sulfide and lanthanum sulfide was discovered in 1976 by Loireau-Lozac’h, Guittard, and Flahut. This family of chalcogenide glasses, referred to as gallium lanthanum sulfide (Ga-La-S) glasses, have a wide region of glass formation centred about the 70Ga2S3:30La2S3 composition and can readily accept other modifiers into their structure. This means that Ga-La-S can be compositionally adjusted to give a wide variety of optical and physical properties. Optically, Ga-La-S has a high refractive index, a transmission window covering most of the visible wavelengths and extending to about 10 µm and a low maximum phonon energy, approx. 450 cm−1. Thermally, the refractive index of Ga-La-S glasses has a strong temperature dependence and low thermal conductivity, which results in strong thermal lensing. However, the high glass transition temperature of Ga-La-S makes it resistant to thermal damage, it has good chemical durability and unlike many chalcogenides which are based on arsenic, its glass components are non-toxic. A clear advantage over other chalcogenides is its high lanthanum content which allows excellent rare-earth solubility and dispersion of the ions in the glass matrix for active devices. Ga-La-S can exist in both glassy and crystalline phases, in a glassy phase, it is a semiconductor with a bandgap of 2.6 eV corresponding to a wavelength of 475 nm; consequently Ga-La-S glass takes a deep orange colour. As with all chalcogenides the phase of the bulk is determined by two key factors; the material composition and the rate at which the molten material is cooled. These variables can be controlled to manipulate the final phase of the material.
When optical fibers are exposed to ionizing radiation such as energetic electrons, protons, neutrons, X-rays, Ƴ-radiation, etc., they undergo 'damage'. The term 'damage' primarily refers to the additional loss of the propagating optical signal leading to decreased power at the output end which could lead to premature failure of the component and or system.
Rigidity theory, or topological constraint theory, is a tool for predicting properties of complex networks based on their composition. It was introduced by Phillips in 1979 and 1981, and refined by Thorpe in 1983. Inspired by the study of the stability of mechanical trusses as pioneered by James Clerk Maxwell, and by the seminal work on glass structure done by William Houlder Zachariasen, this theory reduces complex molecular networks to nodes constrained by rods, thus filtering out microscopic details that ultimately don't affect macroscopic properties. An equivalent theory was developed by P.K. Gupta A.R. Cooper in 1990, where rather than nodes representing atoms, they represented unit polytopes. An example of this would be the SiO tetrahedra in pure glassy silica. This style of analysis has applications in biology and chemistry, such as understanding adaptability in protein-protein interaction networks. Rigidity theory applied to the molecular networks arising from phenotypical expression of certain diseases may provide insights regarding their structure and function.

Lead bismuthate is a superconductor with the formula Pb(BiO3)2. has only been discovered in recent years in the laboratory as it is not naturally occurring. Lead bismuthate forms a pentavalent structure, significantly different from the regular ionic interactions of sodium bismuthate, but similar to that of strontium bismuthate. In the structure, six oxygen atoms are coordinated octahedrally to both the bismuth and lead atoms. The bismuth and oxygen atoms form negatively charged layers by creating repeating octahedral geometries. The positively charged lead atoms are then disbursed within the layers, forming a hexagonal unit cell, with a lead atom in each of the corners. The density of the crystal is 9.18 g/cm3. The formula weight is 233.99 g/mol. The volume of the crystal structure unit is 169.26 A3. Lattice parameters (a) is 5.321 angstroms.
Jacques Lucas, born on June 12, 1937, is Professor Emeritus at the University of Rennes 1. Jacques Lucas is a solids-based chemist who specializes in the discovery of new lenses, contributing to their analysis, knowledge of their optical properties and their use in various fields. He is a member of the French Academy of sciences.









