Taxaceae, commonly called the yew family, is a coniferous family which includes six extant and two extinct genera, and about 30 species of plants, or in older interpretations three genera and 7 to 12 species.

The Pauson–Khand (PK) reaction is a chemical reaction, described as a [2+2+1] cycloaddition. In it, an alkyne, an alkene, and carbon monoxide combine into a α,β-cyclopentenone in the presence of a metal-carbonyl catalyst Ihsan Ullah Khand (1935–1980) discovered the reaction around 1970, while working as a postdoctoral associate with Peter Ludwig Pauson (1925–2013) at the University of Strathclyde in Glasgow. Pauson and Khand's initial findings were intermolecular in nature, but the reaction has poor selectivity. Some modern applications instead apply the reaction for intramolecular ends.
Agelasines are 7,9-dialkylpurinium salts isolated from marine sponges. They are considered secondary metabolites. Their contribution to the sponge is assumed to be some sort of protection against microorganisms. At the present time a total of eleven 9-methyladeninium salts, agelasine A–I, epiagelasine C and agelin B, are known. All compounds carry a diterpenoid side chain in the adenine 7-position. The agelasines are closely related in structure with the agelasimines.
The Bischler–Napieralski reaction is an intramolecular electrophilic aromatic substitution reaction that allows for the cyclization of β-arylethylamides or β-arylethylcarbamates. It was first discovered in 1893 by August Bischler and Bernard Napieralski, in affiliation with Basel Chemical Works and the University of Zurich. The reaction is most notably used in the synthesis of dihydroisoquinolines, which can be subsequently oxidized to isoquinolines.

Taxanes are a class of diterpenes. They were originally identified from plants of the genus Taxus (yews), and feature a taxadiene core. Paclitaxel (Taxol) and docetaxel (Taxotere) are widely used as chemotherapy agents. Cabazitaxel was FDA approved to treat hormone-refractory prostate cancer.

Taxus canadensis, the Canada yew or Canadian yew, is a conifer native to central and eastern North America, thriving in swampy woods, ravines, riverbanks and on lake shores. Locally called simply as "yew", this species is also referred to as American yew or ground-hemlock.

Paclitaxel total synthesis in organic chemistry is a major ongoing research effort in the total synthesis of paclitaxel (Taxol). This diterpenoid is an important drug in the treatment of cancer but, also expensive because the compound is harvested from a scarce resource, namely the Pacific yew. Not only is the synthetic reproduction of the compound itself of great commercial and scientific importance, but it also opens the way to paclitaxel derivatives not found in nature but with greater potential.

C3H4O is a chemical formula that represents each of several actual and hypothetical compounds that differ in structure, but each consist of three atoms of carbon, four of hydrogen, and one of oxygen. The following compounds are among them:
Taxoids are a class of derivatives from taxol, that is, paclitaxel. They were developed for their anticancer chemotherapeutic properties. Taxoids are usually treated as synonymous with taxanes; for example, a major medical dictionary defines the two terms with the same definition phrasing, and in another the phrasing varies slightly but conveys nearly identical meaning.

Wahid Shams-Kolahi is a scientist and an electrical engineer who is known for his research in photovoltaic-related technologies.

Callystatin A is a polyketide natural product from the leptomycin family of secondary metabolites. It was first isolated in 1997 from the marine sponge Callyspongia truncata which was collected from the Goto Islands in the Nagasaki Prefecture of Japan by the Kobayashi group. Since then its absolute configuration has been elucidated and callystatin A was discovered to have anti-fungal and anti-tumor activities with extreme potency against the human epidermoid carcinoma KB cells (IG50 = 10 pg/ml) and the mouse lymphocytic leukemia Ll210 cells (IG50 = 20 pg/ml).

Taxuyunnanines is a class of taxoids isolated from plants of the genus Taxus.
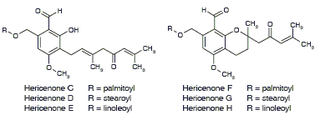
Hericenones is a class of benzaldehydes that are isolates of the fruiting body of Hericium erinaceum that promote nerve growth factor synthesis in vitro.

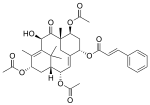
Taxine alkaloids, which are often named under the collective title of taxines, are the toxic chemicals that can be isolated from the yew tree. The amount of taxine alkaloids depends on the species of yew, with Taxus baccata and Taxus cuspidata containing the most. The major taxine alkaloids are taxine A and taxine B although there are at least 10 different alkaloids. Until 1956, it was believed that all the taxine alkaloids were one single compound named taxine.
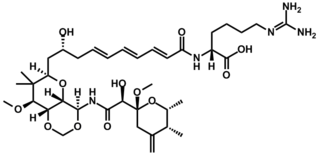
Onnamide A is a bioactive natural product found in Theonella swinhoei, a species of marine sponge whose genus is well known for yielding a diverse set of biologically active natural products, including the swinholides and polytheonamides. It bears structural similarities to the pederins, a family of compounds known to inhibit protein synthesis in eukaryotic cells. Onnamide A and its analogues have attracted academic interest due to their cytotoxicity and potential for combating the growth and proliferation of cancer cells.
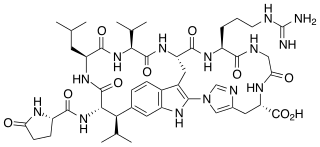
Moroidin is a biologically active compound found in the plants Dendrocnide moroides and Celosia argentea. It is a peptide composed of eight amino acids, with unusual leucine-tryptophan and tryptophan-histidine cross-links that form its two rings. Moroidin has been shown to be at least one of several bioactive compounds responsible for the painful sting of the Dendrocnide moroides plant. It also has demonstrated anti-mitotic properties, specifically by inhibition of tubulin polymerization. Anti-mitotic activity gives moroidin potential as a chemotherapy drug, and this property combined with its unusual chemical structure has made it a target for organic synthesis.
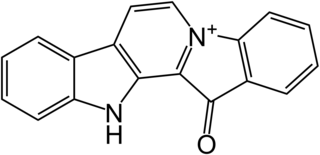
Fascaplysin is a marine alkaloid based on 12H-pyrido[1–2-a:3,4-b′]diindole ring system. It was first isolated as a red pigment from the marine sponge Fascaplysinopsis reticulata that was collected in the South Pacific near Fiji in 1988. Fascaplysin possesses a broad range of in vitro biological activities including analgesic, antimicrobial, antifungal, antiviral, antimalarial, anti-angiogenic, and antiproliferative activity against numerous cancer cell lines.

6-Bromotryptamine is a substituted tryptamine that is a marine natural product.
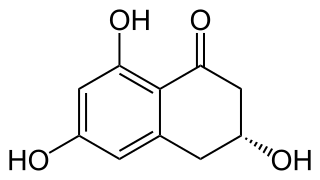
Scytalone or Scytolone is a cyclic beta hydroxy ketone substituted by hydroxy groups at positions 3, 6, and 8. It is a natural product found in various fungal species including Cytospora populina and Ceratocystis fimbriata.

Altohyrtin A is a polyether macrolide originally isolated from the Okinawan marine sponge Hyrtios altum by Kobayashi et al. in 1993, the Indian marine sponge Spongia sp. by Pettit et al. in 1993, and the Japanese marine sponge Cinachyra sp. by Fusetani et al. in 1993. It has potent anti-cancer activity.