The vagus nerve, also known as the tenth cranial nerve, cranial nerve X, or simply CN X, is a cranial nerve that carries sensory fibers that create a pathway that interfaces with the parasympathetic control of the heart, lungs, and digestive tract.

The parasympathetic nervous system is one of the three divisions of the autonomic nervous system, the others being the sympathetic nervous system and the enteric nervous system. The enteric nervous system is sometimes considered part of the autonomic nervous system, and sometimes considered an independent system.

Palpitations are perceived abnormalities of the heartbeat characterized by awareness of cardiac muscle contractions in the chest, which is further characterized by the hard, fast and/or irregular beatings of the heart.

A transcutaneous electrical nerve stimulation is a device that produces mild electric current to stimulate the nerves for therapeutic purposes. TENS, by definition, covers the complete range of transcutaneously applied currents used for nerve excitation, but the term is often used with a more restrictive intent—namely, to describe the kind of pulses produced by portable stimulators used to reduce pain. The unit is usually connected to the skin using two or more electrodes which are typically conductive gel pads. A typical battery-operated TENS unit is able to modulate pulse width, frequency, and intensity. Generally, TENS is applied at high frequency (>50 Hz) with an intensity below motor contraction or low frequency (<10 Hz) with an intensity that produces motor contraction. More recently, many TENS units use a mixed frequency mode which alleviates tolerance to repeated use. Intensity of stimulation should be strong but comfortable with greater intensities, regardless of frequency, producing the greatest analgesia. While the use of TENS has proved effective in clinical studies, there is controversy over which conditions the device should be used to treat.

In human anatomy, the carotid sinus is a dilated area at the base of the internal carotid artery just superior to the bifurcation of the internal carotid and external carotid at the level of the superior border of thyroid cartilage. The carotid sinus extends from the bifurcation to the "true" internal carotid artery. The carotid sinus is sensitive to pressure changes in the arterial blood at this level. It is the major baroreception site in humans and most mammals.

Vagus nerve stimulation (VNS) is a medical treatment that involves delivering electrical impulses to the vagus nerve. It is used as an add-on treatment for certain types of intractable epilepsy, cluster headaches, treatment-resistant depression and stroke rehabilitation.

A jugular foramen is one of the two large foramina (openings) in the base of the skull, located behind the carotid canal. It is formed by the temporal bone and the occipital bone. It allows many structures to pass, including the inferior petrosal sinus, three cranial nerves, the sigmoid sinus, and meningeal arteries.

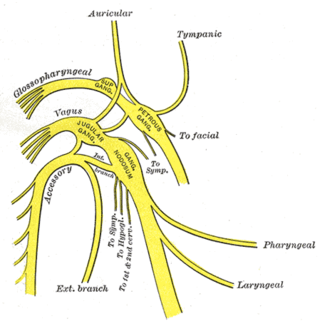
The superior ganglion of the vagus nerve is a sensory ganglion of the peripheral nervous system. It is located within the jugular foramen, where the vagus nerve exits the skull. It is smaller than and proximal to the inferior ganglion of the vagus nerve.
The cough reflex occurs when stimulation of cough receptors in the respiratory tract by dust or other foreign particles produces a cough, which causes rapidly moving air which usually remove the foreign material before it reaches the lungs. This typically clears particles from the bronchi and trachea, the tubes that feed air to lung tissue from the nose and mouth. The larynx and carina are especially sensitive. Cough receptors in the surface cells (epithelium) of the respiratory tract are also sensitive to chemicals. Terminal bronchioles and even the alveoli are sensitive to chemicals such as sulfur dioxide gas or chlorine gas.
The cholinergic anti-inflammatory pathway regulates the innate immune response to injury, pathogens, and tissue ischemia. It is the efferent, or motor arm of the inflammatory reflex, the neural circuit that responds to and regulates the inflammatory response.
A vagal maneuver is an action used to stimulate the parasympathetic nervous system by activating the vagus nerve. The vagus nerve is the longest nerve of the autonomic nervous system and helps regulate many critical aspects of human physiology, including heart rate, blood pressure, sweating, and digestion through the release of acetylcholine. Common maneuvers that activate the vagus nerve include the Valsalva maneuver and carotid sinus massage, which can serve diagnostic or therapeutic functions.
Neural top–down control of physiology concerns the direct regulation by the brain of physiological functions. Cellular functions include the immune system’s production of T-lymphocytes and antibodies, and nonimmune related homeostatic functions such as liver gluconeogenesis, sodium reabsorption, osmoregulation, and brown adipose tissue nonshivering thermogenesis. This regulation occurs through the sympathetic and parasympathetic system, and their direct innervation of body organs and tissues that starts in the brainstem. There is also a noninnervation hormonal control through the hypothalamus and pituitary (HPA). These lower brain areas are under control of cerebral cortex ones. Such cortical regulation differs between its left and right sides. Pavlovian conditioning shows that brain control over basic cell level physiological function can be learned.

Atrial fibrillation is an abnormal heart rhythm (arrhythmia) characterized by rapid and irregular beating of the atrial chambers of the heart. It often begins as short periods of abnormal beating, which become longer or continuous over time. It may also start as other forms of arrhythmia such as atrial flutter that then transform into AF.
Roemheld syndrome (RS), or gastrocardiac syndrome, or gastric cardiac syndrome or Roemheld–Techlenburg–Ceconi syndrome or gastric-cardia, was a medical syndrome first coined by Ludwig von Roemheld (1871–1938) describing a cluster of cardiovascular symptoms stimulated by gastrointestinal changes. Although it is currently considered an obsolete medical diagnosis, recent studies have described similar clinical presentations and highlighted potential underlying mechanisms.

Arrhythmias, also known as cardiac arrhythmias, are irregularities in the heartbeat, including when it is too fast or too slow. A resting heart rate that is too fast – above 100 beats per minute in adults – is called tachycardia, and a resting heart rate that is too slow – below 60 beats per minute – is called bradycardia. Some types of arrhythmias have no symptoms. Symptoms, when present, may include palpitations or feeling a pause between heartbeats. In more serious cases, there may be lightheadedness, passing out, shortness of breath, chest pain, or decreased level of consciousness. While most cases of arrhythmia are not serious, some predispose a person to complications such as stroke or heart failure. Others may result in sudden death.
Neurostimulation is the purposeful modulation of the nervous system's activity using invasive or non-invasive means. Neurostimulation usually refers to the electromagnetic approaches to neuromodulation.
The inflammatory reflex is a neural circuit that regulates the immune response to injury and invasion. All reflexes have an afferent and efferent arc. The Inflammatory reflex has a sensory afferent arc, which is activated by cytokines and a motor or efferent arc, which transmits action potentials in the vagus nerve to suppress cytokine production. Increased signaling in the efferent arc inhibits inflammation and prevents organ damage.

Celivarone is an experimental drug being tested for use in pharmacological antiarrhythmic therapy. Cardiac arrhythmia is any abnormality in the electrical activity of the heart. Arrhythmias range from mild to severe, sometimes causing symptoms like palpitations, dizziness, fainting, and even death. They can manifest as slow (bradycardia) or fast (tachycardia) heart rate, and may have a regular or irregular rhythm.
Neuromodulation is "the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body". It is carried out to normalize – or modulate – nervous tissue function. Neuromodulation is an evolving therapy that can involve a range of electromagnetic stimuli such as a magnetic field (rTMS), an electric current, or a drug instilled directly in the subdural space. Emerging applications involve targeted introduction of genes or gene regulators and light (optogenetics), and by 2014, these had been at minimum demonstrated in mammalian models, or first-in-human data had been acquired. The most clinical experience has been with electrical stimulation.
Drug-resistant epilepsy (DRE), also known as refractory epilepsy, intractable epilepsy, or pharmacoresistant epilepsy, is diagnosed following a failure of adequate trials of two tolerated and appropriately chosen and used antiepileptic drugs (AEDs) to achieve sustained seizure freedom. The probability that the next medication will achieve seizure freedom drops with every failed AED. For example, after two failed AEDs, the probability that the third will achieve seizure freedom is around 4%. Drug-resistant epilepsy is commonly diagnosed after several years of uncontrolled seizures, however, in most cases, it is evident much earlier. Approximately 30% of people with epilepsy have a drug-resistant form.










