Related Research Articles

A protease is an enzyme that catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism, and cell signaling.
Enteropeptidase is an enzyme produced by cells of the duodenum and is involved in digestion in humans and other animals. Enteropeptidase converts trypsinogen into its active form trypsin, resulting in the subsequent activation of pancreatic digestive enzymes. Absence of enteropeptidase results in intestinal digestion impairment.

Suppressor of tumorigenicity 14 protein, also known as matriptase, is a protein that in humans is encoded by the ST14 gene. ST14 orthologs have been identified in most mammals for which complete genome data are available.

Kallikrein-6 is a protein that in humans is encoded by the KLK6 gene. Kallikrein-6 is also referred to as neurosin, protease M, hK6, or zyme. It is a 223 amino acid sequence, derived from its 244 original form, which contains a 16 residue presignal and 5 residue activation peptide.

Kunitz-type protease inhibitor 2 is an enzyme inhibitor that in humans is encoded by the SPINT2 gene. SPINT2 is a transmembrane protein with two extracellular Kunitz domains to inhibit serine proteases. This gene is a presumed tumor suppressor by inhibiting HGF activator which prevents the formation of active hepatocyte growth factor. Mutations in SPINT2 could result in congenital sodium diarrhea (CSD).

Fibroblast activation protein alpha (FAP-alpha) also known as prolyl endopeptidase FAP is an enzyme that in humans is encoded by the FAP gene.
Kunitz-type protease inhibitor 1 is an enzyme that in humans is encoded by the SPINT1 gene.
Kallikrein-related peptidase 7 (KLK7) is a serine protease that in humans is encoded by the KLK7 gene. KLK7 was initially purified from the epidermis and characterised as stratum corneum chymotryptic enzyme (SCCE). It was later identified as the seventh member of the human kallikrein family, which includes fifteen homologous serine proteases located on chromosome 19 (19q13).

Kallikrein-14 is a protein that in humans is encoded by the KLK14 gene.

Prostasin is a protein that in humans is encoded by the PRSS8 gene.

Serine protease hepsin is an enzyme that in humans is encoded by the HPN gene.

Serine/threonine-protein kinase TAO2 is an enzyme that in humans is encoded by the TAOK2 gene.

Corin, also called atrial natriuretic peptide-converting enzyme, is a protein that in humans is encoded by the CORIN gene.

Transmembrane protease, serine 11D is an enzyme that in humans is encoded by the TMPRSS11D gene.
Tryptase beta-2, also known as tryptase II, is a proteolytic enzyme that in humans is encoded by the TPSB2 gene.
Intramembrane proteases (IMPs), also known as intramembrane-cleaving proteases (I-CLiPs), are enzymes that have the property of cleaving transmembrane domains of integral membrane proteins. All known intramembrane proteases are themselves integral membrane proteins with multiple transmembrane domains, and they have their active sites buried within the lipid bilayer of cellular membranes. Intramembrane proteases are responsible for proteolytic cleavage in the cell signaling process known as regulated intramembrane proteolysis (RIP).
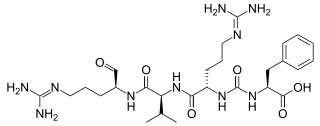
Antipain is an oligopeptide that is isolated from actinomycetes and used in biochemical research as a protease inhibitor of trypsin and papain. It was discovered in 1972 and was the first natural peptide found that contained an ureylene group. Antipain can aid in prevention of coagulation in blood. It is an inhibitor of serine and cysteine proteases.
Mannan-binding lectin-associated serine protease-2 is an enzyme. This enzyme catalyses the following chemical reaction

Protease, serine, 3 is a protein that in humans is encoded by the PRSS3 gene.

The PA clan is the largest group of proteases with common ancestry as identified by structural homology. Members have a chymotrypsin-like fold and similar proteolysis mechanisms but can have identity of <10%. The clan contains both cysteine and serine proteases. PA clan proteases can be found in plants, animals, fungi, eubacteria, archaea and viruses.
References
- ↑ Lee SL, Dickson RB, Lin CY (November 2000). "Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease". The Journal of Biological Chemistry. 275 (47): 36720–5. doi: 10.1074/jbc.M007802200 . PMID 10962009.
- ↑ Lin CY, Anders J, Johnson M, Sang QA, Dickson RB (June 1999). "Molecular cloning of cDNA for matriptase, a matrix-degrading serine protease with trypsin-like activity". The Journal of Biological Chemistry. 274 (26): 18231–6. doi: 10.1074/jbc.274.26.18231 . PMID 10373424.