
Glass is an amorphous (non-crystalline) solid. Because it is often transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass are named after the material, e.g. "glass", "glasses", "magnifying glass".
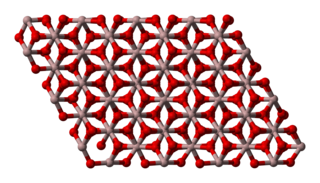
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum in various forms and applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is used to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.

Sodium carbonate is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood, sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the chloralkali process.

Glass fiber is a material consisting of numerous extremely fine fibers of glass.
Sodium silicate is a generic name for chemical compounds with the formula Na
2xSi
yO
2y+x or (Na
2O)
x·(SiO
2)
y, such as sodium metasilicate, sodium orthosilicate, and sodium pyrosilicate. The anions are often polymeric. These compounds are generally colorless transparent solids or white powders, and soluble in water in various amounts.
The Bayer process is the principal industrial means of refining bauxite to produce alumina (aluminium oxide) and was developed by Carl Josef Bayer. Bauxite, the most important ore of aluminium, contains only 30–60% aluminium oxide (Al2O3), the rest being a mixture of silica, various iron oxides, and titanium dioxide. The aluminium oxide must be further purified before it can be refined into aluminium.

Fernico describes a family of metal alloys made primarily of iron, nickel and cobalt. The family includes Kovar, FerNiCo I, FerNiCo II, and Dumet. The name is made up of the chemical symbols of its constituent three elements. "Dumet" is a portmanteau of "dual" and "metal," because it is a heterogeneous alloy, usually fabricated in the form of a wire with an alloy core and a copper cladding. These alloys possess the properties of electrical conductivity, minimal oxidation and formation of porous surfaces at working temperatures of glass and thermal coefficients of expansion which match glass closely. These requirements allow the alloys to be used in glass seals, such that the seal does not crack, fracture or leak with changes in temperature.

In materials science, a refractory is a material that is resistant to decomposition by heat or chemical attack and that retains its strength and rigidity at high temperatures. They are inorganic, non-metallic compounds that may be porous or non-porous, and their crystallinity varies widely: they may be crystalline, polycrystalline, amorphous, or composite. They are typically composed of oxides, carbides or nitrides of the following elements: silicon, aluminium, magnesium, calcium, boron, chromium and zirconium. Many refractories are ceramics, but some such as graphite are not, and some ceramics such as clay pottery are not considered refractory. Refractories are distinguished from the refractory metals, which are elemental metals and their alloys that have high melting temperatures.

Borosilicate glass is a type of glass with silica and boron trioxide as the main glass-forming constituents. Borosilicate glasses are known for having very low coefficients of thermal expansion, making them more resistant to thermal shock than any other common glass. Such glass is subjected to less thermal stress and can withstand temperature differentials without fracturing of about 165 °C (300 °F). It is commonly used for the construction of reagent bottles and flasks, as well as lighting, electronics, and cookware. For many other applications, soda-lime glass is more common.
Sodium oxide is a chemical compound with the formula Na2O. It is used in ceramics and glasses. It is a white solid but the compound is rarely encountered. Instead "sodium oxide" is used to describe components of various materials such as glasses and fertilizers which contain oxides that include sodium and other elements. Sodium oxide is a component.

Bioglass 45S5 or calcium sodium phosphosilicate, is a bioactive glass specifically composed of 45 wt% SiO2, 24.5 wt% CaO, 24.5 wt% Na2O, and 6.0 wt% P2O5. Typical applications of Bioglass 45S5 include: bone grafting biomaterials, repair of periodontal defects, cranial and maxillofacial repair, wound care, blood loss control, stimulation of vascular regeneration, and nerve repair.
In chemistry, an aluminate is a compound containing an oxyanion of aluminium, such as sodium aluminate. In the naming of inorganic compounds, it is a suffix that indicates a polyatomic anion with a central aluminium atom.
Dealkalization is a process of surface modification applicable to glasses containing alkali ions, wherein a thin surface layer is created that has a lower concentration of alkali ions than is present in the underlying, bulk glass. This change in surface composition commonly alters the observed properties of the surface, most notably enhancing corrosion resistance.
Glass production involves two main methods – the float glass process that produces sheet glass, and glassblowing that produces bottles and other containers. It has been done in a variety of ways during the history of glass.
Fluxes are substances, usually oxides, used in glasses, glazes and ceramic bodies to lower the high melting point of the main glass forming constituents, usually silica and alumina. A ceramic flux functions by promoting partial or complete liquefaction. The most commonly used fluxing oxides in a ceramic glaze contain lead, sodium, potassium, lithium, calcium, magnesium, barium, zinc, strontium, and manganese. These are introduced to the raw glaze as compounds, for example lead as lead oxide. Boron is considered by many to be a glass former rather than a flux.

Plate glass, flat glass or sheet glass is a type of glass, initially produced in plane form, commonly used for windows, glass doors, transparent walls, and windscreens. For modern architectural and automotive applications, the flat glass is sometimes bent after production of the plane sheet. Flat glass stands in contrast to container glass and glass fibre.

Container glass is a type of glass for the production of glass containers, such as bottles, jars, drinkware, and bowls. Container glass stands in contrast to flat glass and glass fiber.
Macor is the trademark for a machinable glass-ceramic developed and sold by Corning Inc. It is a white material that looks somewhat like porcelain. Macor is a good thermal insulator and is stable up to temperatures of 1000 °C, with very little thermal expansion or outgassing. It can be machined using standard metalworking tools.
Optical glass refers to a quality of glass suitable for the manufacture of optical systems such as optical lenses, prisms or mirrors. Unlike window glass or crystal, whose formula is adapted to the desired aesthetic effect, optical glass contains additives designed to modify certain optical or mechanical properties of the glass: refractive index, dispersion, transmittance, thermal expansion and other parameters. Lenses produced for optical applications use a wide variety of materials, from silica and conventional borosilicates to elements such as germanium and fluorite, some of which are essential for glass transparency in areas other than the visible spectrum.











