
Glucuronic acid is a uronic acid that was first isolated from urine. It is found in many gums such as gum arabic, xanthan, and kombucha tea and is important for the metabolism of microorganisms, plants and animals.

Cyanidin is a natural organic compound. It is a particular type of anthocyanidin. It is a pigment found in many red berries including grapes, bilberry, blackberry, blueberry, cherry, chokeberry, cranberry, elderberry, hawthorn, loganberry, açai berry and raspberry. It can also be found in other fruits such as apples and plums, and in red cabbage and red onion. It has a characteristic reddish-purple color, though this can change with pH; solutions of the compound are red at pH < 3, violet at pH 7-8, and blue at pH > 11. In certain fruits, the highest concentrations of cyanidin are found in the seeds and skin. Cyanidin has been found to be a potent sirtuin 6 (SIRT6) activator.

Paeonia lactiflora is a species of herbaceous perennial flowering plant in the family Paeoniaceae, native to central and eastern Asia from eastern Tibet across northern China to eastern Siberia.
In enzymology, an anthocyanidin 3-O-glucosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an anthocyanin 3'-O-beta-glucosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a cyanidin 3-O-rutinoside 5-O-glucosyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a flavonol 3-O-glucosyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a galactosylgalactosylxylosylprotein 3-beta-glucuronosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a luteolin-7-O-diglucuronide 4'-O-glucuronosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a luteolin-7-O-glucuronide 2"-O-glucuronosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a luteolin 7-O-glucuronosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acetylgalactosaminyl-proteoglycan 3-beta-glucuronosyltransferase is an enzyme that catalyzes the chemical reaction

Anthocyanins, also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart gave the name Anthokyan to a chemical compound that gives flowers a blue color for the first time in his treatise "Die Farben der Blüthen". Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.
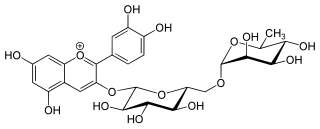
Antirrhinin is an anthocyanin. It is the 3-rutinoside of cyanidin.

Chrysanthemin is an anthocyanin. It is the 3-glucoside of cyanidin.

p-Coumaroylated anthocyanins are a type of anthocyanins with a p-coumaric acid unit linked with a sugar to an anthocyanidin aglycone. 3-(6-p-Coumaroyl)glucosides are found in grape and wine. Cyanidin-3-O-(di-p-coumarylglucoside)-5-glucoside is found in dark opal basil. Red leaves of Perilla frutescens also accumulate cyanidin 3-(6-O-p-coumaroyl-β-D-glucoside)-5-(6-O-malonyl-β-D-glucoside).
Aureusidin synthase is an enzyme with systematic name 2',4,4',6'-tetrahydroxychalcone 4'-O-beta-D-glucoside:oxygen oxidoreductase.
Baicalein 7-O-glucuronosyltransferase is an enzyme with systematic name UDP-D-glucuronate:5,6,7-trihydroxyflavone 7-O-glucuronosyltransferase . This enzyme catalyses the following chemical reaction
Soyasapogenol glucuronosyltransferase is an enzyme with systematic name UDP-D-glucuronate:soyasapogenol 3-O-D-glucuronosyltransferase. This enzyme catalyses the following chemical reaction

Luteolin-7-O-glucuronide is a chemical compound that is classified as a flavone.










