Rutin is the glycoside combining the flavonol quercetin and the disaccharide rutinose. It is a flavonoid glycoside found in a wide variety of plants, including citrus.
In enzymology, a leucocyanidin oxygenase (EC 1.14.11.19) is an enzyme that catalyzes the chemical reaction

Flavonoids are synthesized by the phenylpropanoid metabolic pathway in which the amino acid phenylalanine is used to produce 4-coumaroyl-CoA. This can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalcones, which contain two phenyl rings. Conjugate ring-closure of chalcones results in the familiar form of flavonoids, the three-ringed structure of a flavone. The metabolic pathway continues through a series of enzymatic modifications to yield flavanones → dihydroflavonols → anthocyanins. Along this pathway, many products can be formed, including the flavonols, flavan-3-ols, proanthocyanidins (tannins) and a host of other various polyphenolics.
In enzymology, an anthocyanidin 3-O-glucosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an anthocyanin 3'-O-beta-glucosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a cyanidin 3-O-rutinoside 5-O-glucosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a flavonol-3-O-glucoside glucosyltransferase is an enzyme that catalyzes the chemical reaction
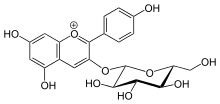
In enzymology, a flavonol-3-O-glucoside L-rhamnosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a flavonol-3-O-glycoside glucosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a flavonol 7-O-beta-glucosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a hydroquinone glucosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an isoflavone 7-O-glucosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a nuatigenin 3beta-glucosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a sarsasapogenin 3β-glucosyltransferase is an enzyme that catalyzes the chemical reaction

Pelargonidin is an anthocyanidin, a type of plant pigment producing a characteristic orange color used in food and industrial dyes.

Anthocyanins, also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart gave the name Anthokyan to a chemical compound that gives flowers a blue color for the first time in his treatise "Die Farben der Blüthen". Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Petunidin (Pt), like Europinidin and Malvidin, is derived from Delphinidin and is an O-methylated anthocyanidin of the 3-hydroxy type. It is a natural organic compound, a dark-red or purple water-soluble pigment found in many redberries including chokeberries, Saskatoon berries or different species of grape, and also part of the pigments responsible for the petal colors in many flowers. This pigment gives the Indigo Rose tomatoes the majority of their deep purple color when the fruits are exposed to sunlight. The name of the molecule itself is derived from the word Petunia.

Laricitrin is an O-methylated flavonol, a type of flavonoid. It is found in red grape and in Vaccinium uliginosum. It is one of the phenolic compounds present in wine.
Anthocyanin 5-O-glucosyltransferase is an enzyme that forms anthocyanin 3,5-O-diglucoside from anthocyanin 3-O-glucoside.