
Glycosyltransferases are enzymes that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar to a nucleophilic glycosyl acceptor molecule, the nucleophile of which can be oxygen- carbon-, nitrogen-, or sulfur-based.

Beta-galactoside alpha-2,6-sialyltransferase 1 is an enzyme that in humans is encoded by the ST6GAL1 gene.
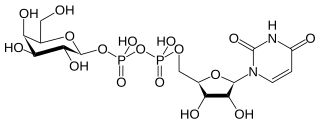
In nucleotide sugar metabolism a group of biochemicals known as nucleotide sugars act as donors for sugar residues in the glycosylation reactions that produce polysaccharides. They are substrates for glycosyltransferases. The nucleotide sugars are also intermediates in nucleotide sugar interconversions that produce some of the activated sugars needed for glycosylation reactions. Since most glycosylation takes place in the endoplasmic reticulum and golgi apparatus, there are a large family of nucleotide sugar transporters that allow nucleotide sugars to move from the cytoplasm, where they are produced, into the organelles where they are consumed.
Nucleotide sugars are the activated forms of monosaccharides. Nucleotide sugars act as glycosyl donors in glycosylation reactions. Those reactions are catalyzed by a group of enzymes called glycosyltransferases.

Desosamine is a 3-(dimethylamino)-3,4,6-trideoxyhexose found in certain macrolide antibiotics such as the commonly prescribed erythromycin, azithromycin, clarithroymcin, methymycin, narbomycin, oleandomycin, picromycin and roxithromycin. As the name suggests, these macrolide antibiotics contain a macrolide or lactone ring and they are attached to the ring Desosamine which is crucial for bactericidal activity. The biological action of the desosamine-based macrolide antibiotics is to inhibit the bacterial ribosomal protein synthesis. These antibiotics which contain Desosamine are widely used to cure bacterial-causing infections in human respiratory system, skin, muscle tissues, and urethra.
In enzymology, an initiation-specific alpha-1,6-mannosyltransferase is an enzyme that catalyzes the chemical reaction in which an alpha-D-mannosyl residue is transferred from GDP-mannose to a lipid-linked oligosaccharide, being linked by an alpha-1,6-D-mannosyl-D-mannose bond.

Dolichyl pyrophosphate Man9GlcNAc2 alpha-1,3-glucosyltransferase is an enzyme that in humans is encoded by the ALG6 gene.

Polypeptide N-acetylgalactosaminyltransferase 1 is an enzyme that in humans is encoded by the GALNT1 gene.

Alpha-1,3/1,6-mannosyltransferase ALG2 is an enzyme that is encoded by the ALG2 gene. Mutations in the human gene are associated with congenital defects in glycosylation The protein encoded by the ALG2 gene belongs to two classes of enzymes: GDP-Man:Man1GlcNAc2-PP-dolichol alpha-1,3-mannosyltransferase and GDP-Man:Man2GlcNAc2-PP-dolichol alpha-1,6-mannosyltransferase.

Probable dolichyl pyrophosphate Glc1Man9GlcNAc2 alpha-1,3-glucosyltransferase is an enzyme that in humans is encoded by the ALG8 gene.

Dolichyl-P-Man:Man(7)GlcNAc(2)-PP-dolichyl-alpha-1,6-mannosyltransferase is an enzyme that in humans is encoded by the ALG12 gene.

Glycosyltransferase-like protein LARGE1 is an enzyme that in humans is encoded by the LARGE gene.
Glycorandomization, is a drug discovery and drug development technology platform to enable the rapid diversification of bioactive small molecules, drug leads and/or approved drugs through the attachment of sugars. Initially developed as a facile method to manipulate carbohydrate substitutions of naturally occurring glycosides to afford the corresponding differentially glycosylated natural product libraries, glycorandomization applications have expanded to include both small molecules and even macromolecules (proteins). Also referred to as 'glycodiversification', glycorandomization has led to the discovery of new glycoside analogs which display improvements in potency, selectivity and/or ADMET as compared to the parent molecule.

Nogalamycin is an anthracycline antibiotic produced by the soil bacteria Streptomyces nogalater. It has antitumor properties but it is also highly cardiotoxic. The less cardiotoxic semisynthetic analog menogaril was developed in the 1970s. Currently nogalamycin and menogaril are not used clinically.
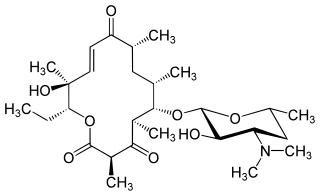
Pikromycin was studied by Brokmann and Hekel in 1951 and was the first antibiotic macrolide to be isolated. Pikromycin is synthesized through a type I polyketide synthase system in Streptomyces venezuelae, a species of Gram-positive bacterium in the genus Streptomyces. Pikromycin is derived from narbonolide, a 14-membered ring macrolide. Along with the narbonolide backbone, pikromycin includes a desosamine sugar and a hydroxyl group. Although Pikromycin is not a clinically useful antibiotic, it can be used as a raw material to synthesize antibiotic ketolide compounds such as ertythromycins and new epothilones.
DTDP-3-amino-3,4,6-trideoxy-alpha-D-glucopyranose N,N-dimethyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:dTDP-3-amino-3,4,6-trideoxy-alpha-D-glucopyranose 3-N,N-dimethyltransferase. This enzyme catalyses the following chemical reaction
DTDP-3-amino-3,6-dideoxy-alpha-D-glucopyranose N,N-dimethyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:dTDP-3-amino-3,6-dideoxy-alpha-D-glucopyranose 3-N,N-dimethyltransferase. This enzyme catalyses the following chemical reaction
Desosaminyl transferase EryCIII is an enzyme with systematic name dTDP-3-dimethylamino-4,6-dideoxy-alpha-D-glucopyranose:3-alpha-mycarosylerythronolide B 3-dimethylamino-4,6-dideoxy-alpha-D-glucosyltransferase. This enzyme catalyses the following chemical reaction
DTDP-3-amino-3,6-dideoxy-alpha-D-glucopyranose transaminase is an enzyme with systematic name dTDP-3-amino-3,6-dideoxy-alpha-D-glucopyranose:2-oxoglutarate aminotransferase. This enzyme catalyses the following chemical reaction
TDP-4-oxo-6-deoxy-alpha-D-glucose-3,4-oxoisomerase (dTDP-3-dehydro-6-deoxy-alpha-D-glucopyranose-forming) is an enzyme with systematic name dTDP-4-dehydro-6-deoxy-alpha-D-glucopyranose:dTDP-3-dehydro-6-deoxy-alpha-D-glucopyranose isomerase. This enzyme catalyses the following chemical reaction











