The putamen is a subcortical nucleus with a rounded structure, in the basal ganglia nuclear group. It is located at the base of the forebrain and above the midbrain.

The striatum or corpus striatum is a cluster of interconnected nuclei that make up the largest structure of the subcortical basal ganglia. The striatum is a critical component of the motor and reward systems; receives glutamatergic and dopaminergic inputs from different sources; and serves as the primary input to the rest of the basal ganglia.

The substantia nigra (SN) is a basal ganglia structure located in the midbrain that plays an important role in reward and movement. Substantia nigra is Latin for "black substance", reflecting the fact that parts of the substantia nigra appear darker than neighboring areas due to high levels of neuromelanin in dopaminergic neurons. Parkinson's disease is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta.

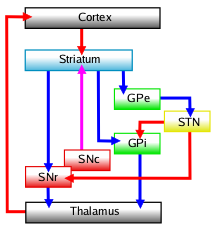
The basal ganglia (BG) or basal nuclei are a group of subcortical nuclei found in the brains of vertebrates. In humans and other primates, differences exist, primarily in the division of the globus pallidus into external and internal regions, and in the division of the striatum. Positioned at the base of the forebrain and the top of the midbrain, they have strong connections with the cerebral cortex, thalamus, brainstem and other brain areas. The basal ganglia are associated with a variety of functions, including regulating voluntary motor movements, procedural learning, habit formation, conditional learning, eye movements, cognition, and emotion.

Deep brain stimulation (DBS) is a surgical procedure that implants a neurostimulator and electrodes which sends electrical impulses to specified targets in the brain responsible for movement control. The treatment is designed for a range of movement disorders such as Parkinson's disease, essential tremor, and dystonia, as well as for certain neuropsychiatric conditions like obsessive-compulsive disorder (OCD) or neurological disorders like epilepsy. The exact mechanisms of DBS are complex and not entirely clear, but it is known to modify brain activity in a structured way.

The globus pallidus (GP), also known as paleostriatum or dorsal pallidum, is a major component of the subcortical basal ganglia in the brain. It consists of two adjacent segments, one external, known in rodents simply as the globus pallidus, and one internal. It is part of the telencephalon, but retains close functional ties with the subthalamus in the diencephalon – both of which are part of the extrapyramidal motor system.

The nigrostriatal pathway is a bilateral dopaminergic pathway in the brain that connects the substantia nigra pars compacta (SNc) in the midbrain with the dorsal striatum in the forebrain. It is one of the four major dopamine pathways in the brain, and is critical in the production of movement as part of a system called the basal ganglia motor loop. Dopaminergic neurons of this pathway release dopamine from axon terminals that synapse onto GABAergic medium spiny neurons (MSNs), also known as spiny projection neurons (SPNs), located in the striatum.

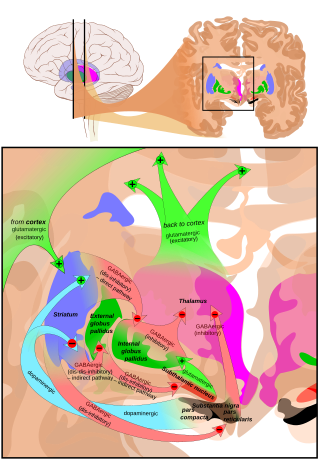
The direct pathway, sometimes known as the direct pathway of movement, is a neural pathway within the central nervous system (CNS) through the basal ganglia which facilitates the initiation and execution of voluntary movement. It works in conjunction with the indirect pathway. Both of these pathways are part of the cortico-basal ganglia-thalamo-cortical loop.

The indirect pathway, sometimes known as the indirect pathway of movement, is a neuronal circuit through the basal ganglia and several associated nuclei within the central nervous system (CNS) which helps to prevent unwanted muscle contractions from competing with voluntary movements. It operates in conjunction with the direct pathway.

In the anatomy of the brain, the centromedian nucleus, also known as the centrum medianum, is a nucleus in the posterior group of the intralaminar thalamic nuclei (ITN) in the thalamus. There are two centromedian nuclei arranged bilaterally.

The subthalamic nucleus (STN) is a small lens-shaped nucleus in the brain where it is, from a functional point of view, part of the basal ganglia system. In terms of anatomy, it is the major part of the subthalamus. As suggested by its name, the subthalamic nucleus is located ventral to the thalamus. It is also dorsal to the substantia nigra and medial to the internal capsule.
Hyperkinesia refers to an increase in muscular activity that can result in excessive abnormal movements, excessive normal movements, or a combination of both. Hyperkinesia is a state of excessive restlessness which is featured in a large variety of disorders that affect the ability to control motor movement, such as Huntington's disease. It is the opposite of hypokinesia, which refers to decreased bodily movement, as commonly manifested in Parkinson's disease.
Hypokinesia is one of the classifications of movement disorders, and refers to decreased bodily movement. Hypokinesia is characterized by a partial or complete loss of muscle movement due to a disruption in the basal ganglia. Hypokinesia is a symptom of Parkinson's disease shown as muscle rigidity and an inability to produce movement. It is also associated with mental health disorders and prolonged inactivity due to illness, amongst other diseases.
The zona incerta (ZI) is a horizontally elongated small nucleus that separates the larger subthalamic nucleus from the thalamus. Its connections project extensively over the brain from the cerebral cortex down into the spinal cord.

The basal ganglia form a major brain system in all vertebrates, but in primates there are special differentiating features. The basal ganglia include the striatum, globus pallidus, substantia nigra and subthalamic nucleus. In primates the pallidus is divided into an external and internal globus pallidus, the external globus pallidus is present in other mammals but not the internal globus pallidus. Also in primates, the dorsal striatum is divided by a large nerve tract called the internal capsule into two masses named the caudate nucleus and the putamen. These differences contribute to a complex circuitry of connections between the striatum and cortex that is specific to primates, reflecting different functions in primate cortical areas.

Medium spiny neurons (MSNs), also known as spiny projection neurons (SPNs), are a special type of inhibitory GABAergic neuron representing approximately 90% of neurons within the human striatum, a basal ganglia structure. Medium spiny neurons have two primary phenotypes : D1-type MSNs of the direct pathway and D2-type MSNs of the indirect pathway. Most striatal MSNs contain only D1-type or D2-type dopamine receptors, but a subpopulation of MSNs exhibit both phenotypes.

The external globus pallidus combines with the internal globus pallidus (GPi) to form the globus pallidus, an anatomical subset of the basal ganglia. Globus pallidus means "pale globe" in Latin, indicating its appearance. The external globus pallidus is the segment of the globus pallidus that is relatively further (lateral) from the midline of the brain.

Basal ganglia disease is a group of physical problems that occur when the group of nuclei in the brain known as the basal ganglia fail to properly suppress unwanted movements or to properly prime upper motor neuron circuits to initiate motor function. Research indicates that increased output of the basal ganglia inhibits thalamocortical projection neurons. Proper activation or deactivation of these neurons is an integral component for proper movement. If something causes too much basal ganglia output, then the ventral anterior (VA) and ventral lateral (VL) thalamocortical projection neurons become too inhibited, and one cannot initiate voluntary movement. These disorders are known as hypokinetic disorders. However, a disorder leading to abnormally low output of the basal ganglia leads to reduced inhibition, and thus excitation, of the thalamocortical projection neurons which synapse onto the cortex. This situation leads to an inability to suppress unwanted movements. These disorders are known as hyperkinetic disorders.

Blocq's disease was first considered by Paul Blocq (1860–1896), who described this phenomenon as the loss of memory of specialized movements causing the inability to maintain an upright posture, despite normal function of the legs in the bed. The patient is able to stand up, but as soon as the feet are on the ground, the patient cannot hold himself upright nor walk; however when lying down, the subject conserved the integrity of muscular force and the precision of movements of the lower limbs. The motivation of this study came when a fellow student Georges Marinesco (1864) and Paul published a case of parkinsonian tremor (1893) due to a tumor located in the substantia nigra.

The cortico-basal ganglia-thalamo-cortical loop is a system of neural circuits in the brain. The loop involves connections between the cortex, the basal ganglia, the thalamus, and back to the cortex. It is of particular relevance to hyperkinetic and hypokinetic movement disorders, such as Parkinson's disease and Huntington's disease, as well as to mental disorders of control, such as attention deficit hyperactivity disorder (ADHD), obsessive–compulsive disorder (OCD), and Tourette syndrome.