| Toluene toxicity | |
|---|---|
 | |
| Chemical structure of toluene | |
| Specialty | Emergency medicine |
Toluene toxicity refers to the harmful effects caused by toluene on the body.
| Toluene toxicity | |
|---|---|
 | |
| Chemical structure of toluene | |
| Specialty | Emergency medicine |
Toluene toxicity refers to the harmful effects caused by toluene on the body.
While a significant amount of toluene, 25%–40%, is exhaled unchanged via the lungs, a greater proportion is metabolised and excreted via other pathways. The primary route of toluene metabolism is by hydroxylation to benzyl alcohol by members of the cytochrome P450 (CYP) superfamily. [1] There are five CYPs which are important in toluene metabolism, CYP1A2, CYP2B6, CYP2E1, CYP2C8, and CYP1A1. [2] The first four seem to be involved in the hydroxylation of toluene to benzyl alcohol. CYP2E1 seems to be the primary enzyme in the hydroxylation of toluene, accounting for roughly 44% of toluene metabolism; [1] however, there is a great deal of ethnic variability, in the Finnish population for example the primary enzyme is CYP2B6. CYP2E1 catalyses the formation of benzyl alcohol and p-cresol, [1] [2] while CYP2B6 produces comparatively little p-cresol. [2]
It is believed that in humans, benzyl alcohol is metabolised to benzaldehyde by CYP rather than alcohol dehydrogenase; [3] however, this belief does not appear to be universal. [4] [5] Benzaldehyde is in turn metabolised to benzoic acid, primarily by mitochondrial aldehyde dehydrogenase-2 (ALDH-2), while only a small percentage is metabolised by cytosolic ALDH-1. [5]
Benzoic acid is metabolised to either benzoyl glucuronide or hippuric acid. [4] [6] Benzoyl glucuronide is produced by the reaction of benzoic acid with glucuronic acid, which accounts for 10–20% of benzoic acid elimination. Hippuric acid is also known as benzoylglycine and is produced from benzoic acid in two steps: first benzoic acid is converted to benzoyl-CoA by the enzyme benzoyl-CoA synthase; then benzoyl-CoA is converted to hippuric acid by benzoyl-CoA:glycine N-acyltransferase. [7] Hippuric acid is the primary urinary metabolite of toluene. [4]

Ring hydroxylation to cresols is a minor pathway in the metabolism of toluene. The majority of the cresol is excreted unchanged in urine; however, some of the p-cresol and o-cresol is excreted as a conjugate. Studies in rats have shown that p-cresol is primarily conjugated with glucuronide to produce p-cresylglucuronide, though this may not be applicable to humans. [8] o-cresol appears to be excreted mostly unchanged in urine or as the glucuronide or sulfate conjugate. [9] There appears to be some dispute over whether m-cresol is produced as a metabolite of toluene or not. [4] [10]

When exposure to toluene occurs there is usually simultaneous exposure to several other chemicals. [4] Often toluene exposure occurs in conjunction with benzene and since they are to some degree metabolised by the same enzymes, the relative concentrations will determine their rate of elimination. [4] Of course the longer it takes for toluene to be eliminated the more harm it is likely to do.
The smoking and drinking habits of those exposed to toluene will partially determine the elimination of toluene. Studies have shown that even a modest amount of acute ethanol consumption can significantly decrease the distribution or elimination of toluene from the blood resulting in increased tissue exposure. [11] Other studies have shown that chronic ethanol consumption can enhance toluene metabolism via the induction of CYP2E1. [12] Smoking has been shown to enhance the elimination rate of toluene from the body, perhaps as a result of enzyme induction. [13]
The diet can also influence toluene elimination. Both a low-carbohydrate diet and fasting have been shown to induce CYP2E1 and as a result increase toluene metabolism. [12] A low protein diet may decrease total CYP content and thereby reduce the elimination rate of the drug. [12]
Hippuric acid has long been used as an indicator of toluene exposure; [14] however, there appears to be some doubt about its validity. [15] There is significant endogenous hippuric acid production by humans; which shows inter- and intra-individual variation influenced by factors such as diet, medical treatment, alcohol consumption, etc. [15] This suggests that hippuric acid may be an unreliable indicator of toluene exposure. [15] [16] It has been suggested that urinary hippuric acid, the traditional marker of toluene exposure is simply not sensitive enough to separate the exposed from the non-exposed. [17] This has led to the investigation of other metabolites as markers for toluene exposure. [16]
Urinary o-cresol may be more reliable for the biomonitoring of toluene exposure because, unlike hippuric acid, o-cresol is not found at detectable levels in unexposed subjects. [16] o-Cresol may be a less sensitive marker of toluene exposure than hippuric acid. [18] o-Cresol excretion may be an unreliable method for measuring toluene exposure because o-cresol makes up <1% of total toluene elimination. [14]
Benzylmercapturic acid, a minor metabolite of toluene, is produced from benzaldehyde. [19] In more recent years, studies have suggested the use of urinary benzylmercapturic acid as the best marker for toluene exposure, because: it is not detected in non-exposed subjects; it is more sensitive than hippuric acid at low concentrations; it is not affected by eating or drinking; it can detect toluene exposure down to approximately 15 ppm; and it shows a better quantitative relationship with toluene than hippuric acid or o-cresol. [20] [21]
Serious adverse behavioural effects are often associated with chronic occupational exposure [22] and toluene abuse related to the deliberate inhalation of solvents. [23] Long-term toluene exposure is often associated with effects such as: psychoorganic syndrome; [24] visual evoked potential (VEP) abnormality; [24] toxic polyneuropathy, cerebellar, cognitive, and pyramidal dysfunctions; [23] [24] optic atrophy; hearing disorders [25] [26] and brain lesions. [23]
The neurotoxic effects of long-term use (in particular repeated withdrawals) of toluene may cause postural tremors by downregulating GABA receptors within the cerebellar cortex. [23] Treatment with GABA agonists such as benzodiazepines provide some relief from toluene-induced tremor and ataxia. [23] An alternative to drug treatment is ventral intermediate nucleus (vim) thalamotomy. [23] The tremors associated with toluene misuse do not seem to be a transient symptom, but an irreversible and progressive symptom which continues after solvent abuse has been discontinued. [23]
There is some evidence that low-level toluene exposure may cause disruption in the differentiation of astrocyte precursor cells. [27] This does not appear to be a major hazard to adults; however, exposure of pregnant women to toluene during critical stages of fetal development could cause serious disruption to neuronal development. [27]

Benzoic acid is a white solid organic compound with the formula C6H5COOH, whose structure consists of a benzene ring with a carboxyl substituent. The benzoyl group is often abbreviated "Bz", thus benzoic acid is also denoted as BzOH, since the benzoyl group has the formula –C6H5CO. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source.

Toluene, also known as toluol, is a substituted aromatic hydrocarbon with the chemical formula C6H5CH3, often abbreviated as PhCH3, where Ph stands for phenyl group. It is a colorless, water-insoluble liquid with the odor associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group by a single bond. As such, its systematic IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent.
Acetonitrile, often abbreviated MeCN, is the chemical compound with the formula CH3CN and structure H3C−C≡N. This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene. The N≡C−C skeleton is linear with a short C≡N distance of 1.16 Å.
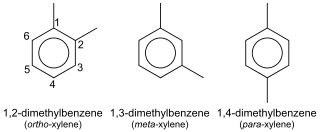
In organic chemistry, xylene or xylol are any of three organic compounds with the formula (CH3)2C6H4. They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are substituted determines which of three structural isomers results. It is a colorless, flammable, slightly greasy liquid of great industrial value.

Benzyl alcohol (also known as α-cresol) is an aromatic alcohol with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful as a solvent for its polarity, low toxicity, and low vapor pressure. Benzyl alcohol has moderate solubility in water (4 g/100 mL) and is miscible in alcohols and diethyl ether. The anion produced by deprotonation of the alcohol group is known as benzylate or benzyloxide.

Sodium benzoate also known as benzoate of soda is the sodium salt of benzoic acid, widely used as a food preservative (with an E number of E211) and a pickling agent. It appears as a white crystalline chemical with the formula C6H5COONa.

Hippuric acid is a carboxylic acid and organic compound. It is found in urine and is formed from the combination of benzoic acid and glycine. Levels of hippuric acid rise with the consumption of phenolic compounds. The phenols are first converted to benzoic acid, and then to hippuric acid and excreted in urine.

Aldrin is an organochlorine insecticide that was widely used until the 1990s, when it was banned in most countries. Aldrin is a member of the so-called "classic organochlorines" (COC) group of pesticides. COCs enjoyed a very sharp rise in popularity during and after World War II. Other noteworthy examples of COCs include dieldrin and DDT. After research showed that organochlorines can be highly toxic to the ecosystem through bioaccumulation, most were banned from use. Before the ban, it was heavily used as a pesticide to treat seed and soil. Aldrin and related "cyclodiene" pesticides became notorious as persistent organic pollutants.

Benzyl benzoate is an organic compound which is used as a medication and insect repellent. As a medication it is used to treat scabies and lice. For scabies either permethrin or malathion is typically preferred. It is applied to the skin as a lotion. Typically two to three applications are needed. It is also present in Balsam of Peru, Tolu balsam, and in a number of flowers.
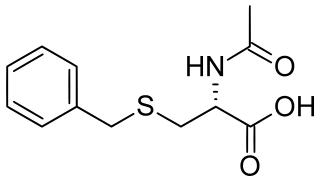
Benzylmercapturic acid is a minor metabolite of toluene in humans and is used in the diagnosis of toluene exposure. As its name indicates, is a benzyl derivative of mercapturic acid (acetylcysteine).

Azinphos-methyl (Guthion) is a broad spectrum organophosphate insecticide manufactured by Bayer CropScience, Gowan Co., and Makhteshim Agan. Like other pesticides in this class, it owes its insecticidal properties to the fact that it is an acetylcholinesterase inhibitor. It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act, and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.
4-Aminobiphenyl (4-ABP) is an organic compound with the formula C6H5C6H4NH2. It is an amine derivative of biphenyl. It is a colorless solid, although aged samples can appear colored. 4-Aminobiphenyl was commonly used in the past as a rubber antioxidant and an intermediate for dyes. Exposure to this aryl-amine can happen through contact with chemical dyes and from inhalation of cigarette smoke. Researches showed that 4-aminobiphenyl is responsible for bladder cancer in humans and dogs by damaging DNA. Due to its carcinogenic effects, commercial production of 4-aminobiphenyl ceased in the United States in the 1950s.

Benzotrichloride (BTC), also known as α,α,α-trichlorotoluene, phenyl chloroform or (trichloromethyl)benzene, is an organic compound with the formula C6H5CCl3. Benzotrichloride is an unstable, colorless or somewhat yellowish, viscous, chlorinated hydrocarbon with a penetrating odor. Benzotrichloride is used extensively as a chemical intermediate for products of various classes, i.e. dyes and antimicrobial agents.

o-Toluidine (ortho-toluidine) is an organic compound with the chemical formula CH3C6H4NH2. It is the most important of the three isomeric toluidines. It is a colorless liquid although commercial samples are often yellowish. It is a precursor to the herbicides metolachlor and acetochlor.

1,1,2,2-tetrachloroethane (TeCA), also known by the brand names Bonoform, Cellon and Westron, is an organic compound. It is colorless liquid and has a sweet odor. It is used as an industrial solvent and as a separation agent. TeCA is toxic and it can be inhaled, consumed or absorbed through the skin. After exposure, nausea, dizziness or even liver damage may occur.

Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon.
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.

Benzoyl-CoA is the thioester derived from benzoic acid and coenzyme A. The term benzoyl-CoA also include diverse conjugates of coenzyme A and aromatic carboxylic acids. Benzoate, vanillin, anthranilic acid, 4-ethylphenol, p-cresol, phenol, aniline, terephthalic acid, [3-hydroxybenzoic acid, and phenylalanine are all metabolized to benzoyl-CoA. Additionally, cinnamic acid, p-coumaric acid, ferulic acid, toluene, caffeic acid, benzyl alcohol, and mandelic acid are suspected to be processed similarly.
ortho-Cresol (IUPAC name: 2-methylphenol, also known as 2-hydroxytoluene or ortho-Toluenol) is an organic compound with the formula CH3C6H4(OH). It is a colourless solid that is widely used intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of p-cresol and m-cresol.

Methylhippuric acid is a carboxylic acid and organic compound. Methylhippuric acid has three isomers. The isomers include 2-, 3-, and 4-methylhippuric acid.
{{cite journal}}: CS1 maint: multiple names: authors list (link)