
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme.

Glycolysis is the metabolic pathway that converts glucose into pyruvate, and in most organisms, occurs in the liquid part of cells, the cytosol. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.

In biochemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology. Protein phosphorylation often activates many enzymes.
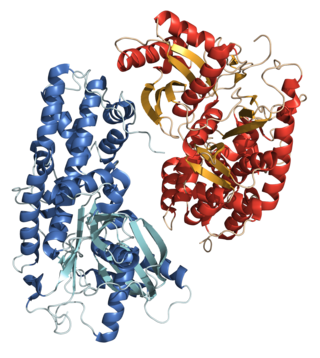
A hexokinase is an enzyme that phosphorylates hexoses, forming hexose phosphate. In most organisms, glucose is the most important substrate for hexokinases, and glucose-6-phosphate is the most important product. Hexokinase possesses the ability to transfer an inorganic phosphate group from ATP to a substrate.

Phosphofructokinase-1 (PFK-1) is one of the most important regulatory enzymes of glycolysis. It is an allosteric enzyme made of 4 subunits and controlled by many activators and inhibitors. PFK-1 catalyzes the important "committed" step of glycolysis, the conversion of fructose 6-phosphate and ATP to fructose 1,6-bisphosphate and ADP. Glycolysis is the foundation for respiration, both anaerobic and aerobic. Because phosphofructokinase (PFK) catalyzes the ATP-dependent phosphorylation to convert fructose-6-phosphate into fructose 1,6-bisphosphate and ADP, it is one of the key regulatory steps of glycolysis. PFK is able to regulate glycolysis through allosteric inhibition, and in this way, the cell can increase or decrease the rate of glycolysis in response to the cell's energy requirements. For example, a high ratio of ATP to ADP will inhibit PFK and glycolysis. The key difference between the regulation of PFK in eukaryotes and prokaryotes is that in eukaryotes PFK is activated by fructose 2,6-bisphosphate. The purpose of fructose 2,6-bisphosphate is to supersede ATP inhibition, thus allowing eukaryotes to have greater sensitivity to regulation by hormones like glucagon and insulin.

Aldolase B also known as fructose-bisphosphate aldolase B or liver-type aldolase is one of three isoenzymes of the class I fructose 1,6-bisphosphate aldolase enzyme, and plays a key role in both glycolysis and gluconeogenesis. The generic fructose 1,6-bisphosphate aldolase enzyme catalyzes the reversible cleavage of fructose 1,6-bisphosphate (FBP) into glyceraldehyde 3-phosphate and dihydroxyacetone phosphate (DHAP) as well as the reversible cleavage of fructose 1-phosphate (F1P) into glyceraldehyde and dihydroxyacetone phosphate. In mammals, aldolase B is preferentially expressed in the liver, while aldolase A is expressed in muscle and erythrocytes and aldolase C is expressed in the brain. Slight differences in isozyme structure result in different activities for the two substrate molecules: FBP and fructose 1-phosphate. Aldolase B exhibits no preference and thus catalyzes both reactions, while aldolases A and C prefer FBP.

Fructose-bisphosphate aldolase, often just aldolase, is an enzyme catalyzing a reversible reaction that splits the aldol, fructose 1,6-bisphosphate, into the triose phosphates dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (G3P). Aldolase can also produce DHAP from other (3S,4R)-ketose 1-phosphates such as fructose 1-phosphate and sedoheptulose 1,7-bisphosphate. Gluconeogenesis and the Calvin cycle, which are anabolic pathways, use the reverse reaction. Glycolysis, a catabolic pathway, uses the forward reaction. Aldolase is divided into two classes by mechanism.
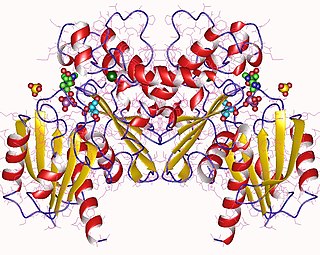
Fructokinase, also known as D-fructokinase or D-fructose (D-mannose) kinase, is an enzyme of the liver, intestine, and kidney cortex. Fructokinase is in a family of enzymes called transferases, meaning that this enzyme transfers functional groups; it is also considered a phosphotransferase since it specifically transfers a phosphate group. Fructokinase specifically catalyzes the transfer of a phosphate group from adenosine triphosphate to fructose as the initial step in its utilization. The main role of fructokinase is in carbohydrate metabolism, more specifically, sucrose and fructose metabolism. The reaction equation is as follows:

In enzymology, 1-phosphofructokinase is an enzyme that catalyzes the chemical reaction
In enzymology, a fucokinase is an enzyme that catalyzes the chemical reaction
In enzymology, a glycerate kinase is an enzyme that catalyzes the chemical reaction

In enzymology, a guanylate kinase is an enzyme that catalyzes the chemical reaction
In enzymology, an inositol-tetrakisphosphate 1-kinase is an enzyme that catalyzes the chemical reaction
In enzymology, an inositol-tetrakisphosphate 5-kinase is an enzyme that catalyzes the chemical reaction
L-Fuculokinase is an enzyme that catalyzes the chemical reaction
In enzymology, a mannokinase is an enzyme that catalyzes the chemical reaction
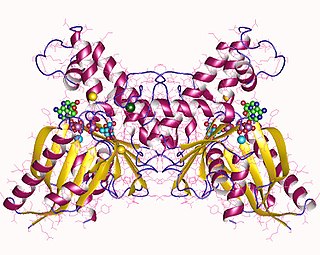
In enzymology, a N-acylmannosamine kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a rhamnulokinase is an enzyme that catalyzes the chemical reaction
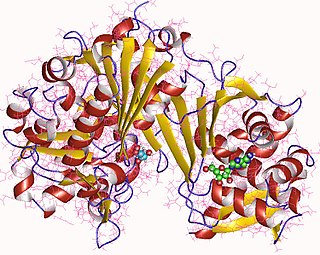
In enzymology, a xylulokinase is an enzyme that catalyzes the chemical reaction
Fructolysis refers to the metabolism of fructose from dietary sources. Though the metabolism of glucose through glycolysis uses many of the same enzymes and intermediate structures as those in fructolysis, the two sugars have very different metabolic fates in human metabolism. Unlike glucose, which is directly metabolized widely in the body, fructose is almost entirely metabolized in the liver in humans, where it is directed toward replenishment of liver glycogen and triglyceride synthesis. Under one percent of ingested fructose is directly converted to plasma triglyceride. 29% - 54% of fructose is converted in liver to glucose, and about a quarter of fructose is converted to lactate. 15% - 18% is converted to glycogen. Glucose and lactate are then used normally as energy to fuel cells all over the body.












