
Nucleotides are organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver.
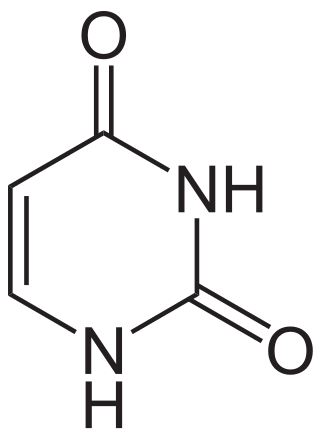
Uracil is one of the four nucleobases in the nucleic acid RNA. The others are adenine (A), cytosine (C), and guanine (G). In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by thymine (T). Uracil is a demethylated form of thymine.
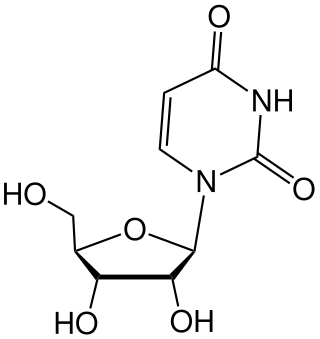
Uridine (symbol U or Urd) is a glycosylated pyrimidine analog containing uracil attached to a ribose ring (or more specifically, a ribofuranose) via a β-N1-glycosidic bond. The analog is one of the five standard nucleosides which make up nucleic acids, the others being adenosine, thymidine, cytidine and guanosine. The five nucleosides are commonly abbreviated to their symbols, U, A, dT, C, and G, respectively. However, thymidine is more commonly written as 'dT' ('d' represents 'deoxy') as it contains a 2'-deoxyribofuranose moiety rather than the ribofuranose ring found in uridine. This is because thymidine is found in deoxyribonucleic acid (DNA) and usually not in ribonucleic acid (RNA). Conversely, uridine is found in RNA and not DNA. The remaining three nucleosides may be found in both RNA and DNA. In RNA, they would be represented as A, C and G whereas in DNA they would be represented as dA, dC and dG.

In biochemistry, a ribonucleotide is a nucleotide containing ribose as its pentose component. It is considered a molecular precursor of nucleic acids. Nucleotides are the basic building blocks of DNA and RNA. Ribonucleotides themselves are basic monomeric building blocks for RNA. Deoxyribonucleotides, formed by reducing ribonucleotides with the enzyme ribonucleotide reductase (RNR), are essential building blocks for DNA. There are several differences between DNA deoxyribonucleotides and RNA ribonucleotides. Successive nucleotides are linked together via phosphodiester bonds.
A salvage pathway is a pathway in which a biological product is produced from intermediates in the degradative pathway of its own or a similar substance. The term often refers to nucleotide salvage in particular, in which nucleotides are synthesized from intermediates in their degradative pathway.
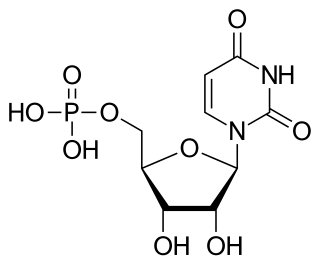
Uridine monophosphate (UMP), also known as 5′-uridylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside uridine. UMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase uracil; hence, it is a ribonucleotide monophosphate. As a substituent or radical its name takes the form of the prefix uridylyl-. The deoxy form is abbreviated dUMP. Covalent attachment of UMP is called uridylylation.

Floxuridine is an oncology drug that belongs to the class known as antimetabolites. Specifically, floxuridine is a pyrimidine analog, classified as a deoxyuridine. The drug is usually administered via an artery, and most often used in the treatment of colorectal cancer. The quality of life and survival rates of individuals that receive continuous hepatic artery infusion of floxuridine for colorectal cancer metastases is significantly higher than control groups. Floxuridine can also be prescribed for the treatment of kidney and stomach cancers. In vitro uses of floxuridine include 5-minute treatments of fluorouracil, floxuridine, and mitomycin to increase cell proliferation in Tenon's capsule fibroblasts.

Purine nucleoside phosphorylase, PNP, PNPase or inosine phosphorylase is an enzyme that in humans is encoded by the NP gene. It catalyzes the chemical reaction

Nucleic acid metabolism is a collective term that refers to the variety of chemical reactions by which nucleic acids are either synthesized or degraded. Nucleic acids are polymers made up of a variety of monomers called nucleotides. Nucleotide synthesis is an anabolic mechanism generally involving the chemical reaction of phosphate, pentose sugar, and a nitrogenous base. Degradation of nucleic acids is a catabolic reaction and the resulting parts of the nucleotides or nucleobases can be salvaged to recreate new nucleotides. Both synthesis and degradation reactions require multiple enzymes to facilitate the event. Defects or deficiencies in these enzymes can lead to a variety of diseases.

Deoxyuridine monophosphate (dUMP), also known as deoxyuridylic acid or deoxyuridylate in its conjugate acid and conjugate base forms, respectively, is a deoxynucleotide.
In enzymology, a pyrimidine-deoxynucleoside 1'-dioxygenase (EC 1.14.11.10) is an enzyme that catalyzes the chemical reaction
In enzymology, a phosphopentomutase is an enzyme that catalyzes the chemical reaction
In enzymology, a S-methyl-5-thioribose-1-phosphate isomerase is an enzyme that catalyzes the chemical reaction

In Enzymology, a dUTP diphosphatase (EC 3.6.1.23) is an enzyme that catalyzes the chemical reaction
In enzymology, an uridine nucleosidase (EC 3.2.2.3) is an enzyme that catalyzes the chemical reaction
In enzymology, a pyrimidine-nucleoside phosphorylase is an enzyme that catalyzes the chemical reaction
In enzymology, a S-methyl-5'-thioadenosine phosphorylase is an enzyme that catalyzes the chemical reaction

Thymidine phosphorylase is an enzyme that is encoded by the TYMP gene and catalyzes the reaction:
In enzymology, an uridine phosphorylase is an enzyme that catalyzes the chemical reaction

In enzymology, an UDP-N-acetylglucosamine diphosphorylase is an enzyme that catalyzes the chemical reaction













