Related Research Articles

Americium is a synthetic chemical element; it has symbol Am and atomic number 95. It is radioactive and a transuranic member of the actinide series in the periodic table, located under the lanthanide element europium and was thus named after the Americas by analogy.

Curium is a synthetic chemical element; it has symbol Cm and atomic number 96. This transuranic actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first intentionally made by the team of Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley. They bombarded the newly discovered element plutonium with alpha particles. This was then sent to the Metallurgical Laboratory at University of Chicago where a tiny sample of curium was eventually separated and identified. The discovery was kept secret until after the end of World War II. The news was released to the public in November 1947. Most curium is produced by bombarding uranium or plutonium with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains ~20 grams of curium.
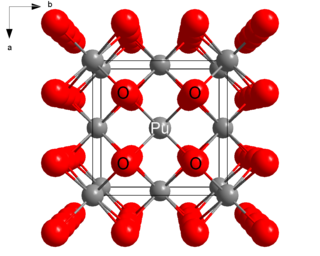
Plutonium(IV) oxide, or plutonia, is a chemical compound with the formula PuO2. This high melting-point solid is a principal compound of plutonium. It can vary in color from yellow to olive green, depending on the particle size, temperature and method of production.
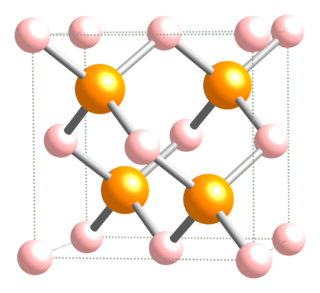
Boron phosphide (BP) (also referred to as boron monophosphide, to distinguish it from boron subphosphide, B12P2) is a chemical compound of boron and phosphorus. It is a semiconductor.
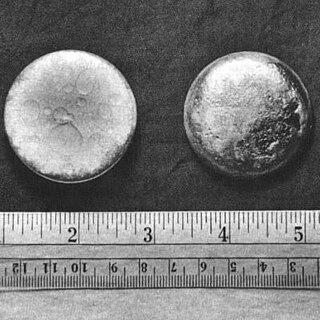
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation states. It reacts with carbon, halogens, nitrogen, silicon, and hydrogen. When exposed to moist air, it forms oxides and hydrides that can expand the sample up to 70% in volume, which in turn flake off as a powder that is pyrophoric. It is radioactive and can accumulate in bones, which makes the handling of plutonium dangerous.

Plutonium hexafluoride is the highest fluoride of plutonium, and is of interest for laser enrichment of plutonium, in particular for the production of pure plutonium-239 from irradiated uranium. This isotope of plutonium is needed to avoid premature ignition of low-mass nuclear weapon designs by neutrons produced by spontaneous fission of plutonium-240.

Yttrium phosphide is an inorganic compound of yttrium and phosphorus with the chemical formula YP. The compound may be also classified as yttrium(III) phosphide.
Plutonium selenide is a binary inorganic compound of plutonium and selenium with the chemical formula PuSe. The compound forms black crystals and does not dissolve in water.
Plutonium silicide is a binary inorganic compound of plutonium and silicon with the chemical formula PuSi. The compound forms gray crystals.
Europium phosphide is an inorganic compound of europium and phosphorus with the chemical formula EuP. Other phosphides are also known.
Samarium(III) phosphide is an inorganic compound of samarium and phosphorus with the chemical formula SmP.
Ytterbium(III) phosphide is an inorganic compound of ytterbium and phosphorus with the chemical formula YbP. This is one of the phosphides of ytterbium.
Holmium phosphide is a binary inorganic compound of holmium and phosphorus with the chemical formula HoP. The compound forms dark crystals and does not dissolve in water.

Dysprosium phosphide is an inorganic compound of dysprosium and phosphorus with the chemical formula DyP.

Terbium phosphide is an inorganic compound of terbium and phosphorus with the chemical formula TbP.
Gadolinium phosphide is an inorganic compound of gadolinium and phosphorus with the chemical formula GdP.
Plutonium arsenide is a binary inorganic compound of plutonium and arsenic with the formula PuAs.
Neptunium compounds are compounds containg the element neptunium (Np). Neptunium has five ionic oxidation states ranging from +3 to +7 when forming chemical compounds, which can be simultaneously observed in solutions. It is the heaviest actinide that can lose all its valence electrons in a stable compound. The most stable state in solution is +5, but the valence +4 is preferred in solid neptunium compounds. Neptunium metal is very reactive. Ions of neptunium are prone to hydrolysis and formation of coordination compounds.
Berkelium(III) phosphide is a binary inorganic compound of berkelium and phosphorus with the chemical formula BkP.
Platinum diphosphide is a binary inorganic compound of platinum metal and phosphorus with the chemical formula PtP2.
References
- ↑ Lam, D. J.; Fradin, F. Y.; Kruger, O. O. (10 November 1969). "Magnetic Properties of Plutonium Monophosphide". Physical Review . 187 (2): 606–610. Bibcode:1969PhRv..187..606L. doi:10.1103/PhysRev.187.606. ISSN 0031-899X.
- ↑ Nuclear Science Abstracts. Oak Ridge Directed Operations, Technical Information Division. 1969. Retrieved 10 January 2022.
- ↑ Fundamental Nuclear Energy Research. United States Atomic Energy Commission. 1964. p. 235. Retrieved 10 January 2022.
- ↑ Reactor Fuel Processing. U.S. Argonne National Laboratory. 1964. p. 188. Retrieved 10 January 2022.
- ↑ "mp-926: PuP (cubic, Fm-3m, 225)". materialsproject.org . Retrieved 10 January 2022.
- ↑ NBS Monograph. National Bureau of Standards. 1959. p. 65. Retrieved 10 January 2022.
- ↑ Gorum, A. E. (10 February 1957). "The crystal structures of PuAs, PuTe, PuP and PuOSe". Acta Crystallographica . 10 (2): 144. doi:10.1107/S0365110X5700047X.