In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells.

Waxes are a diverse class of organic compounds that are lipophilic, malleable solids near ambient temperatures. They include higher alkanes and lipids, typically with melting points above about 40 °C (104 °F), melting to give low viscosity liquids. Waxes are insoluble in water but soluble in nonpolar organic solvents such as hexane, benzene and chloroform. Natural waxes of different types are produced by plants and animals and occur in petroleum.
A deodorant is a substance applied to the body to prevent or mask body odor caused by bacterial breakdown of perspiration, for example in the armpits, groin, or feet. A subclass of deodorants, called antiperspirants, prevents sweating itself, typically by blocking sweat glands. Antiperspirants are used on a wider range of body parts, at any place where sweat would be inconvenient or unsafe, since unwanted sweating can interfere with comfort, vision, and grip. Other types of deodorant allow sweating but prevent bacterial action on sweat, since human sweat only has a noticeable smell when it is decomposed by bacteria.

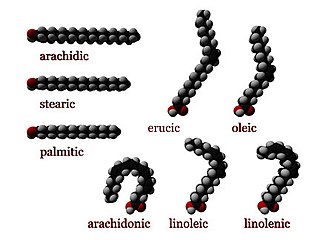
Stearic acid is a saturated fatty acid with an 18-carbon chain. The IUPAC name is octadecanoic acid. It is a soft waxy solid with the formula CH3(CH2)16CO2H. The triglyceride derived from three molecules of stearic acid is called stearin. Stearic acid is a prevalent fatty-acid in nature, found in many animal and vegetable fats, but is usually higher in animal fat than vegetable fat. It has a melting point of 69.4 °C (156.9 °F) °C and a pKa of 4.50.

Hair conditioner is a hair care cosmetic product used to improve the feel, texture, appearance and manageability of hair. Its main purpose is to reduce friction between strands of hair to allow smoother brushing or combing, which might otherwise cause damage to the scalp. Various other benefits are often advertised, such as hair repair, strengthening, or a reduction in split ends.

Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, is a silicone polymer with a wide variety of uses, from cosmetics to industrial lubrication and passive daytime radiative cooling.

A moisturizer, or emollient, is a cosmetic preparation used for protecting, moisturizing, and lubricating the skin. These functions are normally performed by sebum produced by healthy skin. The word "emollient" is derived from the Latin verb mollire, to soften.

Oleyl alcohol, or cis-9-octadecen-1-ol, is an unsaturated fatty alcohol with the molecular formula C18H36O or the condensed structural formula CH3(CH2)7−CH=CH−(CH2)8OH. It is a colorless oil, mainly used in cosmetics.
Stearyl heptanoate is the ester of stearyl alcohol and heptanoic acid. It is used in cosmetics, including eyeliner. It is prepared from stearyl alcohol, which may be derived from animal or vegetable sources.
Fatty alcohols (or long-chain alcohols) are usually high-molecular-weight, straight-chain primary alcohols, but can also range from as few as 4–6 carbons to as many as 22–26, derived from natural fats and oils. The precise chain length varies with the source. Some commercially important fatty alcohols are lauryl, stearyl, and oleyl alcohols. They are colourless oily liquids (for smaller carbon numbers) or waxy solids, although impure samples may appear yellow. Fatty alcohols usually have an even number of carbon atoms and a single alcohol group (–OH) attached to the terminal carbon. Some are unsaturated and some are branched. They are widely used in industry. As with fatty acids, they are often referred to generically by the number of carbon atoms in the molecule, such as "a C12 alcohol", that is an alcohol having 12 carbons, for example dodecanol.

A Langmuir–Blodgett (LB) film is a nanostructured system formed when Langmuir films—or Langmuir monolayers (LM)—are transferred from the liquid-gas interface to solid supports during the vertical passage of the support through the monolayers. LB films can contain one or more monolayers of an organic material, deposited from the surface of a liquid onto a solid by immersing the solid substrate into the liquid. A monolayer is adsorbed homogeneously with each immersion or emersion step, thus films with very accurate thickness can be formed. This thickness is accurate because the thickness of each monolayer is known and can therefore be added to find the total thickness of a Langmuir–Blodgett film.
Cetyl alcohol, also known as hexadecan-1-ol and palmityl alcohol, is a C-16 fatty alcohol with the formula CH3(CH2)15OH. At room temperature, cetyl alcohol takes the form of a waxy white solid or flakes. The name cetyl derives from the whale oil (cetacea oil, from Latin: cetus, lit. 'whale', from Ancient Greek: κῆτος, romanized: kētos, lit. 'huge fish') from which it was first isolated.

Panthenol (also called pantothenol) is the alcohol analog of pantothenic acid (vitamin B5), and is thus a provitamin of B5. In organisms, it is quickly oxidized to pantothenic acid. It is a viscous transparent liquid at room temperature. Panthenol is used in pharmaceutical and cosmetic products as a moisturizer and to improve wound healing.

In cosmetics, skin toner or simply toner refers to a lotion, tonic or wash designed to cleanse the skin and shrink the appearance of pores, usually used on the face. It also moisturizes, protects and refreshes the skin. Toners can be applied to the skin in different ways:
Hydrolyzed jojoba esters are the hydrolysate of jojoba esters derived by acid, enzyme or other method of hydrolysis. Hydrolyzed jojoba esters are commonly used in cosmetic formulations.
Cetrimonium chloride, or cetyltrimethylammonium chloride (CTAC), is a topical antiseptic and surfactant. Long-chain quaternary ammonium surfactants, such as cetyltrimethylammonium chloride (CTAC), are generally combined with long-chain fatty alcohols, such as stearyl alcohols, in formulations of hair conditioners and shampoos. The cationic surfactant concentration in conditioners is generally of the order of 1–2% and the alcohol concentrations are usually equal to or greater than those of the cationic surfactants. The ternary system, surfactant/fatty alcohol/water, leads to a lamellar structure forming a percolated network giving rise to a gel.

Cetostearyl alcohol, cetearyl alcohol or cetylstearyl alcohol is a mixture of fatty alcohols, consisting predominantly of cetyl and stearyl alcohols and is classified as a fatty alcohol. It is used as an emulsion stabilizer, opacifying agent, and foam boosting surfactant, as well as an aqueous and nonaqueous viscosity-increasing agent. It imparts an emollient feel to the skin and can be used in water-in-oil emulsions, oil-in-water emulsions, and anhydrous formulations. It is commonly used in hair conditioners and other hair products.

Stearyl palmityl tartrate is a derivative of tartaric acid used as an emulsifier. It is produced by esterification of tartaric acid with commercial grade stearyl alcohol, which generally consists of a mixture of the fatty alcohols stearyl and palmityl alcohol. Stearyl palmityl tartrate consists mainly of diesters, with minor amounts of monoester and of unchanged starting materials.

Evaporation suppressing monolayers are materials that when applied to the air/water interface, will spread and form a thin film across the surface of the water. The purpose of these materials is to reduce evaporative water loss from dams and reservoirs.

Hair oil is an oil-based cosmetic product intended to improve the condition of hair. Various types of oils may be included in hair oil products. These often purport to aid with hair growth, dryness, or damage.













