
Nitric acid is the inorganic compound with the formula HNO3. It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%.

Sodium nitrate is the chemical compound with the formula NaNO
3. This alkali metal nitrate salt is also known as Chile saltpeter to distinguish it from ordinary saltpeter, potassium nitrate. The mineral form is also known as nitratine, nitratite or soda niter.

Silver nitrate is an inorganic compound with chemical formula AgNO
3. It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called lunar caustic because silver was called luna by ancient alchemists who associated silver with the moon. In solid silver nitrate, the silver ions are three-coordinated in a trigonal planar arrangement.

Copper(II) nitrate describes any member of the family of inorganic compounds with the formula Cu(NO3)2(H2O)x. The hydrates are blue solids. Anhydrous copper nitrate forms blue-green crystals and sublimes in a vacuum at 150-200 °C. Common hydrates are the hemipentahydrate and trihydrate.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.
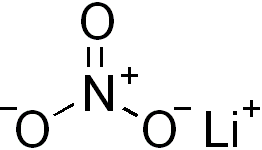
Lithium nitrate is an inorganic compound with the formula LiNO3. It is the lithium salt of nitric acid (an alkali metal nitrate). The salt is deliquescent, absorbing water to form the hydrated form, lithium nitrate trihydrate. Its eutectics are of interest for heat transfer fluids.

Calcium nitrate are inorganic compounds with the formula Ca(NO3)2(H2O)x. The anhydrous compound, which is rarely encountered, absorbs moisture from the air to give the tetrahydrate. Both anhydrous and hydrated forms are colourless salts. Hydrated calcium nitrate, also called Norgessalpeter (Norwegian salpeter), is mainly used as a component in fertilizers, but it has other applications. Nitrocalcite is the name for a mineral which is a hydrated calcium nitrate that forms as an efflorescence where manure contacts concrete or limestone in a dry environment as in stables or caverns. A variety of related salts are known including calcium ammonium nitrate decahydrate and calcium potassium nitrate decahydrate.
Rubidium nitrate is an inorganic compound with the formula RbNO3. This alkali metal nitrate salt is white and highly soluble in water.

Zinc nitrate is an inorganic chemical compound with the formula Zn(NO3)2. This colorless, crystalline salt is highly deliquescent. It is typically encountered as a hexahydrate Zn(NO3)2·6H2O. It is soluble in both water and alcohol.
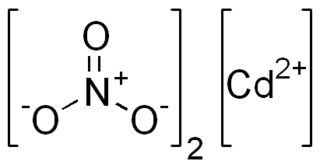
Cadmium nitrate describes any of the related members of a family of inorganic compounds with the general formula , the most commonly encountered form being the tetrahydrate. The anhydrous form is volatile, but the others are colourless crystalline solids that are deliquescent, tending to absorb enough moisture from the air to form an aqueous solution. Like other cadmium compounds, cadmium nitrate is known to be carcinogenic.

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.

Cobalt nitrate is the inorganic compound with the formula Co(NO3)2.xH2O. It is cobalt(II)'s salt. The most common form is the hexahydrate Co(NO3)2·6H2O, which is a red-brown deliquescent salt that is soluble in water and other polar solvents.

Mercury(I) nitrate is an inorganic compound, a salt of mercury and nitric acid with the formula Hg2(NO3)2. A yellow solid, the compound is used as a precursor to other Hg22+ complexes. The structure of the hydrate has been determined by X-ray crystallography. It consists of a [H2O-Hg-Hg-OH2]2+ center, with a Hg-Hg distance of 254 pm.
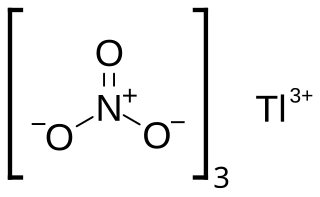
Thallium(III) nitrate, also known as thallic nitrate, is a thallium compound with chemical formula Tl(NO3)3. It is normally found as the trihydrate. It is a colorless and highly toxic salt. It is a strong oxidizing agent useful in organic synthesis. Among its many transformations, it oxidizes methoxyl phenols to quinone acetals, alkenes to acetals, and cyclic alkenes to ring-contracted aldehydes.

Cerium nitrate refers to a family of nitrates of cerium in the +3 or +4 oxidation state. Often these compounds contain water, hydroxide, or hydronium ions in addition to cerium and nitrate. Double nitrates of cerium also exist.

Zirconium nitrate is a volatile anhydrous transition metal nitrate salt of zirconium with formula Zr(NO3)4. It has alternate names of zirconium tetranitrate, or zirconium(IV) nitrate.

Thorium(IV) nitrate is a chemical compound, a salt of thorium and nitric acid with the formula Th(NO3)4. A white solid in its anhydrous form, it can form tetra- and pentahydrates. As a salt of thorium it is weakly radioactive.
Indium(III) nitrate is a nitrate salt of indium which forms various hydrates. Only the pentahydrate has been crystallographically verified. Other hydrates are also reported in literature, such as the trihydrate.

Iron(II) nitrate is the nitrate salt of iron(II). It is commonly encountered as the green hexahydrate, Fe(NO3)2·6H2O, which is a metal aquo complex, however it is not commercially available unlike iron(III) nitrate due to its instability to air. The salt is soluble in water serves as a ready source of ferrous ions.
Tin(IV) acetate is the acetate salt of tin(IV), with the chemical formula of Sn(CH3COO)4.























