
In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a P–O–P linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate (Na2H2P2O7) and tetrasodium pyrophosphate (Na4P2O7), among others. Often pyrophosphates are called diphosphates. The parent pyrophosphates are derived from partial or complete neutralization of pyrophosphoric acid. The pyrophosphate bond is also sometimes referred to as a phosphoanhydride bond, a naming convention which emphasizes the loss of water that occurs when two phosphates form a new P–O–P bond, and which mirrors the nomenclature for anhydrides of carboxylic acids. Pyrophosphates are found in ATP and other nucleotide triphosphates, which are important in biochemistry. The term pyrophosphate is also the name of esters formed by the condensation of a phosphorylated biological compound with inorganic phosphate, as for dimethylallyl pyrophosphate. This bond is also referred to as a high-energy phosphate bond.
A polyphosphate is a salt or ester of polymeric oxyanions formed from tetrahedral PO4 (phosphate) structural units linked together by sharing oxygen atoms. Polyphosphates can adopt linear or a cyclic ring structures. In biology, the polyphosphate esters ADP and ATP are involved in energy storage. A variety of polyphosphates find application in mineral sequestration in municipal waters, generally being present at 1 to 5 ppm. GTP, CTP, and UTP are also nucleotides important in the protein synthesis, lipid synthesis, and carbohydrate metabolism, respectively. Polyphosphates are also used as food additives, marked E452.

In enzymology, a glucose 1-dehydrogenase (EC 1.1.1.47) is an enzyme that catalyzes the chemical reaction
In enzymology, a bis(5'-nucleosyl)-tetraphosphatase (asymmetrical) (EC 3.6.1.17) is an enzyme that catalyzes the chemical reaction
In enzymology, a bis(5'-nucleosyl)-tetraphosphatase (symmetrical) (EC 3.6.1.41) is an enzyme that catalyzes the chemical reaction
In enzymology, a diphosphoinositol-polyphosphate diphosphatase (EC 3.6.1.52) is an enzyme that catalyzes the chemical reaction
In enzymology, a K+-transporting ATPase (EC 3.6.3.12) is an enzyme that catalyzes the chemical reaction
In enzymology, a m7G(5')pppN diphosphatase (EC 3.6.1.30) is an enzyme that catalyzes the chemical reaction

In enzymology, a nucleoside-diphosphatase (EC 3.6.1.6) is an enzyme that catalyzes the chemical reaction
In enzymology, a phosphoadenylylsulfatase (EC 3.6.2.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a trimetaphosphatase (EC 3.6.1.2) is an enzyme that catalyzes the chemical reaction

The enzyme diisopropyl-fluorophosphatase (EC 3.1.8.2) catalyzes the reaction
In enzymology, an ADP deaminase (EC 3.5.4.7) is an enzyme that catalyzes the chemical reaction
Decylcitrate synthase (EC 2.3.3.2) is an enzyme that catalyzes the chemical reaction in enzymology.
In enzymology, a polyphosphate kinase, or polyphosphate polymerase, is an enzyme that catalyzes the formation of polyphosphate from ATP, with chain lengths of up to a thousand or more orthophosphate moieties.

Iodine pentoxide is the chemical compound with the formula I2O5. This iodine oxide is the anhydride of iodic acid, and the only stable oxide of iodine. It is produced by dehydrating iodic acid at 200 °C in a stream of dry air:
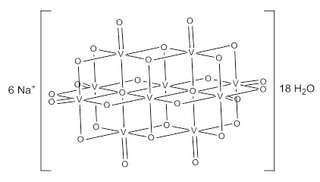
Sodium decavanadate describes any member of the family of inorganic compounds with the formula Na6[V10O28](H2O)n. These are sodium salts of the orange-colored decavanadate anion [V10O28]6−. Numerous other decavanadate salts have been isolated and studied since 1956 when it was first characterized.

The cyanonickelates are a class of chemical compound containing anions consisting of nickel atoms, and cyanide groups. The most important of these are the tetracyanonickelates containing four cyanide groups per nickel. The tetracyanonickelates contain the [Ni(CN)4]2− anion. This can exist in solution or in solid salts. The ion has cyanide groups arranged in a square around the central nickel ion. The symmetry of the ion is D4h. The distance from the nickel atom to the carbon is 1.87 Å, and the carbon-nitrogen distance is 1.16 Å. Tetracyanonickelate(II) can be oxidised electrochemically in solution to yield tetracyanonickelate(III) [Ni(CN)4]−. [Ni(CN)4]− is unstable and Ni(III) oxidises the cyanide to cyanate OCN−. Tetracyanonickelate(III) can add two more cyanide groups to form hexacyanonickelate(III).
Gösta Pettersson is an emeritus professor in biochemistry at Lund University, Sweden. He was born in 1937 in Varberg, Sweden. He gained his Ph.D. at Lund University in 1966 on the basis of a thesis on toluquinones, and his early research was mainly concerned with fumigatin and other products of fungal metabolism.

A transition metal nitrate complex is a coordination compound containing one or more nitrate ligands. Such complexes are common starting reagents for the preparation of other compounds.








