
Plant hormones are signal molecules, produced within plants, that occur in extremely low concentrations. Plant hormones control all aspects of plant growth and development, including embryogenesis, the regulation of organ size, pathogen defense, stress tolerance and reproductive development. Unlike in animals each plant cell is capable of producing hormones. Went and Thimann coined the term "phytohormone" and used it in the title of their 1937 book.

Trichomes are fine outgrowths or appendages on plants, algae, lichens, and certain protists. They are of diverse structure and function. Examples are hairs, glandular hairs, scales, and papillae. A covering of any kind of hair on a plant is an indumentum, and the surface bearing them is said to be pubescent.

Jasmonate (JA) and its derivatives are lipid-based plant hormones that regulate a wide range of processes in plants, ranging from growth and photosynthesis to reproductive development. In particular, JAs are critical for plant defense against herbivory and plant responses to poor environmental conditions and other kinds of abiotic and biotic challenges. Some JAs can also be released as volatile organic compounds (VOCs) to permit communication between plants in anticipation of mutual dangers.

Abscisic acid is a plant hormone. ABA functions in many plant developmental processes, including seed and bud dormancy, the control of organ size and stomatal closure. It is especially important for plants in the response to environmental stresses, including drought, soil salinity, cold tolerance, freezing tolerance, heat stress and heavy metal ion tolerance.

Phytoalexins are antimicrobial substances, some of which are antioxidative as well. They are defined not by their having any particular chemical structure or character, but by the fact that they are defensively synthesized de novo by plants that produce the compounds rapidly at sites of pathogen infection. In general phytoalexins are broad spectrum inhibitors; they are chemically diverse, and different chemical classes of compounds are characteristic of particular plant taxa. Phytoalexins tend to fall into several chemical classes, including terpenoids, glycosteroids, and alkaloids; however, the term applies to any phytochemicals that are induced by microbial infection.

The apoplast is the extracellular space outside of plant cell membranes, especially the fluid-filled cell walls of adjacent cells where water and dissolved material can flow and diffuse freely. Fluid and material flows occurring in any extracellular space are called apoplastic flow or apoplastic transport. The apoplastic pathway is one route by which water and solutes are transported and distributed to different places through tissues and organs, contrasting with the symplastic pathway.

Methyl jasmonate is a volatile organic compound used in plant defense and many diverse developmental pathways such as seed germination, root growth, flowering, fruit ripening, and senescence. Methyl jasmonate is derived from jasmonic acid and the reaction is catalyzed by S-adenosyl-L-methionine:jasmonic acid carboxyl methyltransferase.
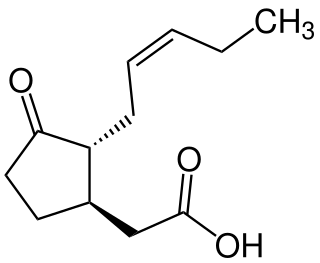
Jasmonic acid (JA) is an organic compound found in several plants including jasmine. The molecule is a member of the jasmonate class of plant hormones. It is biosynthesized from linolenic acid by the octadecanoid pathway. It was first isolated in 1957 as the methyl ester of jasmonic acid by the Swiss chemist Édouard Demole and his colleagues.

Plant defense against herbivory or host-plant resistance is a range of adaptations evolved by plants which improve their survival and reproduction by reducing the impact of herbivores. Many plants produce secondary metabolites, known as allelochemicals, that influence the behavior, growth, or survival of herbivores. These chemical defenses can act as repellents or toxins to herbivores or reduce plant digestibility. Another defensive strategy of plants is changing their attractiveness. Plants can sense being touched, and they can respond with strategies to defend against herbivores. Plants alter their appearance by changing their size or quality in a way that prevents overconsumption by large herbivores, reducing the rate at which they are consumed.
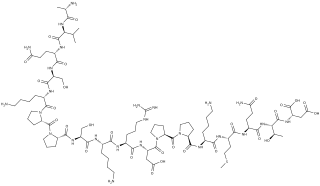
Systemin is a plant peptide hormone involved in the wound response in the family Solanaceae. It was the first plant hormone that was proven to be a peptide having been isolated from tomato leaves in 1991 by a group led by Clarence A. Ryan. Since then, other peptides with similar functions have been identified in tomato and outside of the Solanaceae. Hydroxyproline-rich glycopeptides were found in tobacco in 2001 and AtPeps were found in Arabidopsis thaliana in 2006. Their precursors are found both in the cytoplasm and cell walls of plant cells, upon insect damage, the precursors are processed to produce one or more mature peptides. The receptor for systemin was first thought to be the same as the brassinolide receptor but this is now uncertain. The signal transduction processes that occur after the peptides bind are similar to the cytokine-mediated inflammatory immune response in animals. Early experiments showed that systemin travelled around the plant after insects had damaged the plant, activating systemic acquired resistance, now it is thought that it increases the production of jasmonic acid causing the same result. The main function of systemins is to coordinate defensive responses against insect herbivores but they also affect plant development. Systemin induces the production of protease inhibitors which protect against insect herbivores, other peptides activate defensins and modify root growth. They have also been shown to affect plants' responses to salt stress and UV radiation. AtPEPs have been shown to affect resistance against oomycetes and may allow A. thaliana to distinguish between different pathogens. In Nicotiana attenuata, some of the peptides have stopped being involved in defensive roles and instead affect flower morphology.

Callose is a plant polysaccharide. Its production is due to the glucan synthase-like gene (GLS) in various places within a plant. It is produced to act as a temporary cell wall in response to stimuli such as stress or damage. Callose is composed of glucose residues linked together through β-1,3-linkages, and is termed a β-glucan. It is thought to be manufactured at the cell wall by callose synthases and is degraded by β-1,3-glucanases. Callose is very important for the permeability of plasmodesmata (Pd) in plants; the plant's permeability is regulated by plasmodesmata callose (PDC). PDC is made by callose synthases and broken down by β-1,3-glucanases (BGs). The amount of callose that is built up at the plasmodesmatal neck, which is brought about by the interference of callose synthases (CalSs) and β-1,3-glucanases, determines the conductivity of the plasmodesmata.

Leucyl aminopeptidases are enzymes that preferentially catalyze the hydrolysis of leucine residues at the N-terminus of peptides and proteins. Other N-terminal residues can also be cleaved, however. LAPs have been found across superkingdoms. Identified LAPs include human LAP, bovine lens LAP, porcine LAP, Escherichia coli LAP, and the solanaceous-specific acidic LAP (LAP-A) in tomato.
Plants have evolved many defense mechanisms against insect herbivory in the 350 million years in which they have co-evolved. Such defenses can be broadly classified into two categories: (1) permanent, constitutive defenses, and (2) temporary, inducible defenses. These differ in that constitutive defenses are present before an herbivore attacks, while induced defenses are activated only when attacks occur. In addition to constitutive defenses, initiation of specific defense responses to herbivory is an important strategy for plant persistence and survival.
Biotic stress is stress that occurs as a result of damage done to an organism by other living organisms, such as bacteria, viruses, fungi, parasites, beneficial and harmful insects, weeds, and cultivated or native plants. It is different from abiotic stress, which is the negative impact of non-living factors on the organisms such as temperature, sunlight, wind, salinity, flooding and drought. The types of biotic stresses imposed on an organism depend the climate where it lives as well as the species' ability to resist particular stresses. Biotic stress remains a broadly defined term and those who study it face many challenges, such as the greater difficulty in controlling biotic stresses in an experimental context compared to abiotic stress.

β-Aminobutyric acid (BABA) is an isomer of the amino acid aminobutyric acid with the chemical formula C4H9NO2. It has two isomers, α-aminobutyric acid and γ-aminobutyric acid (GABA), a neurotransmitter in animals that is also found in plants, where it may play a role in signalling. All three are non-proteinogenic amino acids, not being found in proteins. BABA is known for its ability to induce plant disease resistance, as well as increased resistance to abiotic stresses, when applied to plants.

Tritrophic interactions in plant defense against herbivory describe the ecological impacts of three trophic levels on each other: the plant, the herbivore, and its natural enemies. They may also be called multitrophic interactions when further trophic levels, such as soil microbes, endophytes, or hyperparasitoids are considered. Tritrophic interactions join pollination and seed dispersal as vital biological functions which plants perform via cooperation with animals.
WRKY transcription factors are proteins that bind DNA. They are transcription factors that regulate many processes in plants and algae (Viridiplantae), such as the responses to biotic and abiotic stresses, senescence, seed dormancy and seed germination and some developmental processes but also contribute to secondary metabolism.
Induced systemic resistance (ISR) is a resistance mechanism in plants that is activated by infection. Its mode of action does not depend on direct killing or inhibition of the invading pathogen, but rather on increasing physical or chemical barrier of the host plant. Like the Systemic Acquired Resistance (SAR) a plant can develop defenses against an invader such as a pathogen or parasite if an infection takes place. In contrast to SAR, which is triggered by the accumulation of salicylic acid, ISR instead relies on signal transduction pathways activated by jasmonate and ethylene.
In plant biology, proteinase inhibitors are a family of small proteins that serve an integral role in the plant’s defense mechanisms against herbivory from insects or microorganisms that may compromise the integrity of the plant.

Cannabis (/ˈkænəbɪs/) is commonly known as marijuana or hemp and has two known strains: Cannabis sativa and Cannabis indica, both of which produce chemicals to deter herbivory. The chemical composition includes specialized terpenes and cannabinoids, mainly tetrahydrocannabinol (THC), and cannabidiol (CBD). These substances play a role in defending the plant from pathogens including insects, fungi, viruses and bacteria. THC and CBD are stored mostly in the trichomes of the plant, and can cause psychological and physical impairment in the user, via the endocannabinoid system and unique receptors. THC increases dopamine levels in the brain, which attributes to the euphoric and relaxed feelings cannabis provides. As THC is a secondary metabolite, it poses no known effects towards plant development, growth, and reproduction. However, some studies show secondary metabolites such as cannabinoids, flavonoids, and terpenes are used as defense mechanisms against biotic and abiotic environmental stressors.














