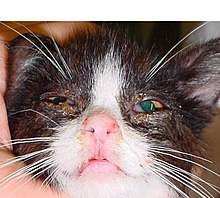
Feline immunodeficiency virus (FIV) is a Lentivirus that affects cats worldwide, with 2.5% to 4.4% of felines being infected.

Feline leukemia virus (FeLV) is a retrovirus that infects cats. FeLV can be transmitted from infected cats when the transfer of saliva or nasal secretions is involved. If not defeated by the animal's immune system, the virus weakens the cat's immune system, which can lead to diseases which can be lethal. Because FeLV is cat-to-cat contagious, FeLV+ cats should only live with other FeLV+ cats.

Carnivore protoparvovirus 1 is a species of parvovirus that infects carnivorans. It causes a highly contagious disease in both dogs and cats separately. The disease is generally divided into two major genogroups: FPV containing the classical feline panleukopenia virus (FPLV), and CPV-2 containing the canine parvovirus type 2 (CPV-2) which appeared in the 1970s.
Pneumonia is an irritation of the lungs caused by different sources. It is characterized by an inflammation of the deep lung tissues and the bronchi. Pneumonia can be acute or chronic. This life-threatening illness is more common in cats than in dogs and the complication “Kennel Cough” can occur in young pets.
Canid alphaherpesvirus 1 (CaHV-1), formerly Canine herpesvirus (CHV), is a virus of the family Herpesviridae which most importantly causes a fatal hemorrhagic disease in puppies less than two to three weeks old. It is known to exist in the United States, Canada, Australia, Japan, England and Germany. CHV was first recognized in the mid-1960s from a fatal disease in puppies.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word ἕρπειν, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. As of 2020, 115 species are recognized, all but one of which are in one of the three subfamilies. Herpesviruses can cause both latent and lytic infections.
Bovine alphaherpesvirus 1 (BoHV-1) is a virus of the family Herpesviridae and the subfamily Alphaherpesvirinae, known to cause several diseases worldwide in cattle, including rhinotracheitis, vaginitis, balanoposthitis, abortion, conjunctivitis, and enteritis. BoHV-1 is also a contributing factor in shipping fever, also known as bovine respiratory disease (BRD). It is spread horizontally through sexual contact, artificial insemination, and aerosol transmission and it may also be transmitted vertically across the placenta. BoHV-1 can cause both clinical and subclinical infections, depending on the virulence of the strain. Although these symptoms are mainly non-life-threatening it is an economically important disease as infection may cause a drop in production and affect trade restrictions. Like other herpesviruses, BoHV-1 causes a lifelong latent infection and sporadic shedding of the virus. The sciatic nerve and trigeminal nerve are the sites of latency. A reactivated latent carrier is normally the source of infection in a herd. The clinical signs displayed are dependent on the virulence of the strain. There is a vaccine available which reduces the severity and incidence of disease. Some countries in Europe have successfully eradicated the disease by applying a strict culling policy.
Aujeszky's disease, usually called pseudorabies in the United States, is a viral disease in swine that is endemic in most parts of the world. It is caused by Suid herpesvirus 1 (SuHV-1). Aujeszky's disease is considered to be the most economically important viral disease of swine in areas where classical swine fever has been eradicated. Other mammals, such as cattle, sheep, goats, cats, dogs, and raccoons, are also susceptible. The disease is usually fatal in these animal species.
Equid alphaherpesvirus 4, formerly Equine herpesvirus 4 (EHV-4) is a virus of the family Herpesviridae that cause rhinopneumonitis in horses. It is the most important viral cause of respiratory infection in foals. Like other herpes viruses, EHV-4 causes a lifelong latent infection in affected animals. These horses are usually the source for new infection for foals over two months old, weanlings, and yearlings. Symptoms include fever, loss of appetite, and discharge from the nose. Most infected animals recover in one to three weeks, but death can occur in environments with overcrowding and other stress factors. There are several vaccines available.
Equid alphaherpesvirus 1, formerly Equine herpesvirus 1 (EHV-1), is a virus of the family Herpesviridae that causes abortion, respiratory disease and occasionally neonatal mortality in horses. Initial spread of EHV-1 by a newly introduced horse through direct and indirect contact can lead to abortion and perinatal infection in up to 70 percent of a previously unexposed herd. Abortion usually occurs in the last four months of gestation, two to four weeks after infection of the mare. Perinatal infection can lead to pneumonia and death. Encephalitis can occur in affected animals, leading to ataxia, paralysis, and death. There is a vaccine available, however its efficacy is questionable. The virus varies in severity from sub-clinical to very severe. Most horses have been infected with EHV-1, but the virus can become latent and persist without ever causing signs of infection. In 2006, an outbreak of EHV-1 among stables in Florida resulted in the institution of quarantine measures. The outbreak was determined to have originated in horses imported from Europe via New York, before being shipped to Florida.

Feline calicivirus (FCV) is a virus of the family Caliciviridae that causes disease in cats. It is one of the two important viral causes of respiratory infection in cats, the other being Felid alphaherpesvirus 1. FCV can be isolated from about 50% of cats with upper respiratory infections. Cheetahs are the other species of the family Felidae known to become infected naturally.

Duck plague is a worldwide disease caused by Anatid alphaherpesvirus 1 (AnHV-1) of the family Herpesviridae that causes acute disease with high mortality rates in flocks of ducks, geese, and swans. It is spread both vertically and horizontally—through contaminated water and direct contact. Migratory waterfowl are a major factor in the spread of this disease as they are often asymptomatic carriers of disease. The incubation period is three to seven days. Birds as young as one week old can be infected. DEV is not zoonotic.

Bovine malignant catarrhal fever (BMCF) is a fatal lymphoproliferative disease caused by a group of ruminant gamma herpes viruses including Alcelaphine gammaherpesvirus 1 (AlHV-1) and Ovine gammaherpesvirus 2 (OvHV-2) These viruses cause unapparent infection in their reservoir hosts, but are usually fatal in cattle and other ungulates such as deer, antelope, and buffalo. In Southern Africa the disease is known as snotsiekte, from the Afrikaans.
Cat flu is the common name for a feline upper respiratory disease, which can be caused by one or more possible pathogens:
- Feline herpes virus, causing feline viral rhinotracheitis,
- Feline calicivirus,
- Bordetella bronchiseptica, or
- Chlamydia felis (chlamydia).

The health of domestic cats is a well studied area in veterinary medicine.
Feline vaccination is animal vaccination applied to cats. Vaccination plays a vital role in protecting cats from infectious diseases, some of which are potentially fatal. They can be exposed to these diseases from their environment, other pets, or even humans.
Salmonid herpesvirus 3 (SalHV-3) is a species of virus in the genus Salmonivirus, family Alloherpesviridae, and order Herpesvirales.
Feline corneal sequestrum is the development of dark areas of dead tissue in the cornea of domestic cats. This disease is painful to the cat, although it develops slowly over a longer period of time. Cats will usually demonstrate teary eye(s), squinting or closing of the eye(s), and covering of the third eyelid.
Recombinant feline interferon omega (RFeIFN-ω), sold under the brand name Virbagen Omega among others, is a recombinant version of a cat interferon alpha. It is used to treat a range of viral diseases in cats and dogs, including canine parvovirus, feline leukemia virus (FeLV), and feline immunodeficiency virus (FIV) in many countries. It is approved to be used by injection under the skin. RFeIFN-ω is produced in silkworm larvae using a baculovirus vector.