
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.

In cell biology, the spindle apparatus is the cytoskeletal structure of eukaryotic cells that forms during cell division to separate sister chromatids between daughter cells. It is referred to as the mitotic spindle during mitosis, a process that produces genetically identical daughter cells, or the meiotic spindle during meiosis, a process that produces gametes with half the number of chromosomes of the parent cell.
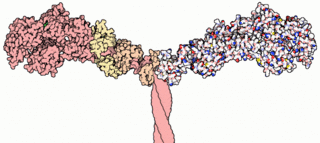
Myosins are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility.
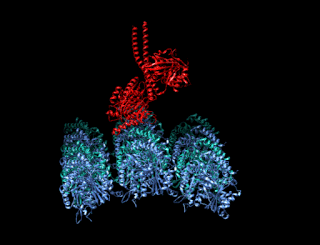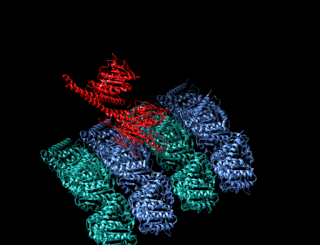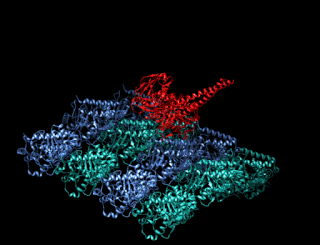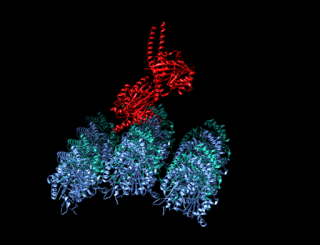
A kinesin is a protein belonging to a class of motor proteins found in eukaryotic cells. Kinesins move along microtubule (MT) filaments and are powered by the hydrolysis of adenosine triphosphate (ATP). The active movement of kinesins supports several cellular functions including mitosis, meiosis and transport of cellular cargo, such as in axonal transport, and intraflagellar transport. Most kinesins walk towards the plus end of a microtubule, which, in most cells, entails transporting cargo such as protein and membrane components from the center of the cell towards the periphery. This form of transport is known as anterograde transport. In contrast, dyneins are motor proteins that move toward the minus end of a microtubule in retrograde transport.

Dyneins are a family of cytoskeletal motor proteins that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella. All of these functions rely on dynein's ability to move towards the minus-end of the microtubules, known as retrograde transport; thus, they are called "minus-end directed motors". In contrast, most kinesin motor proteins move toward the microtubules' plus-end, in what is called anterograde transport.
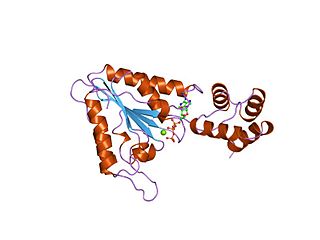
AAA proteins or ATPases Associated with diverse cellular Activities are a protein family sharing a common conserved module of approximately 230 amino acid residues. This is a large, functionally diverse protein family belonging to the AAA+ protein superfamily of ring-shaped P-loop NTPases, which exert their activity through the energy-dependent remodeling or translocation of macromolecules.

Molecular motors are natural (biological) or artificial molecular machines that are the essential agents of movement in living organisms. In general terms, a motor is a device that consumes energy in one form and converts it into motion or mechanical work; for example, many protein-based molecular motors harness the chemical free energy released by the hydrolysis of ATP in order to perform mechanical work. In terms of energetic efficiency, this type of motor can be superior to currently available man-made motors. One important difference between molecular motors and macroscopic motors is that molecular motors operate in the thermal bath, an environment in which the fluctuations due to thermal noise are significant.

Motor proteins are a class of molecular motors that can move along the cytoplasm of cells. They convert chemical energy into mechanical work by the hydrolysis of ATP. Flagellar rotation, however, is powered by a proton pump.
The Kinesin-13 Family are a subfamily of motor proteins known as kinesins. Most kinesins transport materials or cargo around the cell while traversing along microtubule polymer tracks with the help of ATP-hydrolysis-created energy.
Plus-end-directed kinesin ATPase (EC 3.6.4.4, kinesin) is an enzyme with systematic name kinesin ATP phosphohydrolase (plus-end-directed). This enzyme catalyses the following chemical reaction

Kinesin-like protein KIF23 is a protein that in humans is encoded by the KIF23 gene.

Kinesin-like protein KIF3A is a protein that in humans is encoded by the KIF3A gene.

Kinesin-like protein KIF3B is a protein that in humans is encoded by the KIF3B gene. KIF3B is an N-type protein that complexes with two other kinesin proteins to form two-headed anterograde motors. First, KIF3B forms a heterodimer with KIF3A ; (KIF3A/3B), that is membrane-bound and has ATPase activity. Then KIFAP3 binds to the tail domain to form a heterotrimeric motor. This motor has a plus end-directed microtubule sliding activity that exhibits a velocity of ~0.3 μm/s a. There are 14 kinesin protein families in the kinesin superfamily and KIF3B is part of the Kinesin-2 family, of kinesins that can all form heterotrimeric complexes. Expression of the three motor subunits is ubiquitous. The KIG3A/3B/KAP3 motors can transport 90 to 160 nm in diameter organelles.

Kinesin-like protein KIF1A, also known as axonal transporter of synaptic vesicles or microtubule-based motor KIF1A, is a protein that in humans is encoded by the KIF1A gene.

Kinesin-like protein KIF11 is a molecular motor protein that is essential in mitosis. In humans it is coded for by the gene KIF11. Kinesin-like protein KIF11 is a member of the kinesin superfamily, which are nanomotors that move along microtubule tracks in the cell. Named from studies in the early days of discovery, it is also known as Kinesin-5, or as BimC, Eg5 or N-2, based on the founding members of this kinesin family.

Ronald David Vale ForMemRS is an American biochemist and cell biologist. He is a professor at the Department of Cellular and Molecular Pharmacology, University of California, San Francisco. His research is focused on motor proteins, particularly kinesin and dynein. He was awarded the Canada Gairdner International Award for Biomedical Research in 2019, the Shaw Prize in Life Science and Medicine in 2017 together with Ian Gibbons, and the Albert Lasker Award for Basic Medical Research in 2012 alongside Michael Sheetz and James Spudich. He is a fellow of the American Academy of Arts and Sciences and a member of the National Academy of Sciences. He was the president of the American Society for Cell Biology in 2012. He has also been an investigator at the Howard Hughes Medical Institute since 1995. In 2019, Vale was named executive director of the Janelia Research Campus and a vice president of HHMI, his appointment began in early 2020.

Neurotubules are microtubules found in neurons in nervous tissues. Along with neurofilaments and microfilaments, they form the cytoskeleton of neurons. Neurotubules are undivided hollow cylinders that are made up of tubulin protein polymers and arrays parallel to the plasma membrane in neurons. Neurotubules have an outer diameter of about 23 nm and an inner diameter, also known as the central core, of about 12 nm. The wall of the neurotubules is about 5 nm in width. There is a non-opaque clear zone surrounding the neurotubule and it is about 40 nm in diameter. Like microtubules, neurotubules are greatly dynamic and the length of them can be adjusted by polymerization and depolymerization of tubulin.
Edwin W. Taylor is an adjunct professor of cell and developmental biology at Northwestern University. He was elected to the National Academy of Sciences in 2001. Taylor received a BA in physics and chemistry from the University of Toronto in 1952; an MSc in physical chemistry from McMaster University in 1955, and a PhD in biophysics from the University of Chicago in 1957. In 2001 Taylor was elected to the National Academy of Scineces in Cellular and Developmental Biology and Biochemistry.
J. Richard McIntosh is a Distinguished Professor Emeritus in Molecular, Cellular, and Developmental Biology at the University of Colorado Boulder. McIntosh first graduated from Harvard with a BA in Physics in 1961, and again with a Ph.D. in Biophysics in 1968. He began his teaching career at Harvard but has spent most of his career at the University of Colorado Boulder. At the University of Colorado Boulder, McIntosh taught biology courses at both the undergraduate and graduate levels. Additionally, he created an undergraduate course in the biology of cancer towards the last several years of his teaching career. McIntosh's research career looks at a variety of things, including different parts of mitosis, microtubules, and motor proteins.

Kinesin family member 25 (KIF25), also known as kinesin-14, is a human protein encoded by the KIF25 gene. It is part of the kinesin family of motor proteins.
















