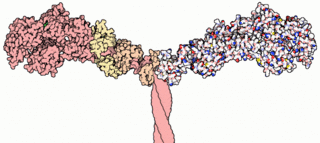
Myosins are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility.
In biochemistry, dephosphorylation is the removal of a phosphate (PO43−) group from an organic compound by hydrolysis. It is a reversible post-translational modification. Dephosphorylation and its counterpart, phosphorylation, activate and deactivate enzymes by detaching or attaching phosphoric esters and anhydrides. A notable occurrence of dephosphorylation is the conversion of ATP to ADP and inorganic phosphate.

Vacuolar-type ATPase (V-ATPase) is a highly conserved evolutionarily ancient enzyme with remarkably diverse functions in eukaryotic organisms. V-ATPases acidify a wide array of intracellular organelles and pumps protons across the plasma membranes of numerous cell types. V-ATPases couple the energy of ATP hydrolysis to proton transport across intracellular and plasma membranes of eukaryotic cells. It is generally seen as the polar opposite of ATP synthase because ATP synthase is a proton channel that uses the energy from a proton gradient to produce ATP. V-ATPase however, is a proton pump that uses the energy from ATP hydrolysis to produce a proton gradient.
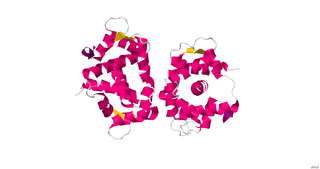
Myosin light-chain kinase also known as MYLK or MLCK is a serine/threonine-specific protein kinase that phosphorylates a specific myosin light chain, namely, the regulatory light chain of myosin II.

Transforming protein RhoA, also known as Ras homolog family member A (RhoA), is a small GTPase protein in the Rho family of GTPases that in humans is encoded by the RHOA gene. While the effects of RhoA activity are not all well known, it is primarily associated with cytoskeleton regulation, mostly actin stress fibers formation and actomyosin contractility. It acts upon several effectors. Among them, ROCK1 and DIAPH1 are the best described. RhoA, and the other Rho GTPases, are part of a larger family of related proteins known as the Ras superfamily, a family of proteins involved in the regulation and timing of cell division. RhoA is one of the oldest Rho GTPases, with homologues present in the genomes since 1.5 billion years. As a consequence, RhoA is somehow involved in many cellular processes which emerged throughout evolution. RhoA specifically is regarded as a prominent regulatory factor in other functions such as the regulation of cytoskeletal dynamics, transcription, cell cycle progression and cell transformation.

ROCK1 is a protein serine/threonine kinase also known as rho-associated, coiled-coil-containing protein kinase 1. Other common names are ROKβ and P160ROCK. ROCK1 is a major downstream effector of the small GTPase RhoA and is a regulator of the actomyosin cytoskeleton which promotes contractile force generation. ROCK1 plays a role in cancer and in particular cell motility, metastasis, and angiogenesis.

Protein kinase C epsilon type (PKCε) is an enzyme that in humans is encoded by the PRKCE gene. PKCε is an isoform of the large PKC family of protein kinases that play many roles in different tissues. In cardiac muscle cells, PKCε regulates muscle contraction through its actions at sarcomeric proteins, and PKCε modulates cardiac cell metabolism through its actions at mitochondria. PKCε is clinically significant in that it is a central player in cardioprotection against ischemic injury and in the development of cardiac hypertrophy.
In enzymology, a myosin-heavy-chain kinase is an enzyme that catalyzes the chemical reaction

Plasma membrane calcium-transporting ATPase 4 is an enzyme that in humans is encoded by the ATP2B4 gene.

26S protease regulatory subunit S10B, also known as 26S proteasome AAA-ATPase subunit Rpt4, is an enzyme that in humans is encoded by the PSMC6 gene. This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.

Myosin-10 also known as myosin heavy chain 10 or non-muscle myosin IIB (NM-IIB) is a protein that in humans is encoded by the MYH10 gene. Non-muscle myosins are expressed in a wide variety of tissues, but NM-IIB is the only non-muscle myosin II isoform expressed in cardiac muscle, where it localizes to adherens junctions within intercalated discs. NM-IIB is essential for normal development of cardiac muscle and for integrity of intercalated discs. Mutations in MYH10 have been identified in patients with left atrial enlargement.

Transcriptional enhancer factor TEF-1 also known as TEA domain family member 1 (TEAD1) and transcription factor 13 (TCF-13) is a protein that in humans is encoded by the TEAD1 gene. TEAD1 was the first member of the TEAD family of transcription factors to be identified.

Myosin essential light chain (ELC), ventricular/cardiac isoform is a protein that in humans is encoded by the MYL3 gene. This cardiac ventricular/slow skeletal ELC isoform is distinct from that expressed in fast skeletal muscle (MYL1) and cardiac atrial muscle (MYL4). Ventricular ELC is part of the myosin molecule and is important in modulating cardiac muscle contraction.

Myosin X, also known as MYO10, is a protein that in humans is encoded by the MYO10 gene.

V-type proton ATPase subunit e 1 is an enzyme that in humans is encoded by the ATP6V0E1 gene.
The Walker A and Walker B motifs are protein sequence motifs, known to have highly conserved three-dimensional structures. These were first reported in ATP-binding proteins by Walker and co-workers in 1982.
Diacylglycerol diphosphate phosphatase (EC 3.1.3.81, DGPP phosphatase, DGPP phosphohydrolase, DPP1, DPPL1, DPPL2, PAP2, pyrophosphate phosphatase) is an enzyme with systematic name 1,2-diacyl-sn-glycerol 3-phosphate phosphohydrolase. This enzyme catalyses the following chemical reaction
Steroid-transporting ATPase (EC 3.6.3.45, pleiotropic-drug-resistance protein, PDR protein) is an enzyme with systematic name ATP phosphohydrolase (steroid-exporting). This enzyme catalyses the following chemical reaction

Calponin 2 is a protein that in humans is encoded by the CNN2 gene.
Edwin W. Taylor is an adjunct professor of cell and developmental biology at Northwestern University. He was elected to the National Academy of Sciences in 2001. Taylor received a BA in physics and chemistry from the University of Toronto in 1952; an MSc in physical chemistry from McMaster University in 1955, and a PhD in biophysics from the University of Chicago in 1957. In 2001 Taylor was elected to the National Academy of Scineces in Cellular and Developmental Biology and Biochemistry.













