
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme.

ATPases (EC 3.6.1.3, Adenosine 5'-TriPhosphatase, adenylpyrophosphatase, ATP monophosphatase, triphosphatase, SV40 T-antigen, ATP hydrolase, complex V (mitochondrial electron transport), (Ca2+ + Mg2+)-ATPase, HCO3−-ATPase, adenosine triphosphatase) are a class of enzymes that catalyze the decomposition of ATP into ADP and a free phosphate ion or the inverse reaction. This dephosphorylation reaction releases energy, which the enzyme (in most cases) harnesses to drive other chemical reactions that would not otherwise occur. This process is widely used in all known forms of life.
Translocase is a general term for a protein that assists in moving another molecule, usually across a cell membrane. These enzymes catalyze the movement of ions or molecules across membranes or their separation within membranes. The reaction is designated as a transfer from “side 1” to “side 2” because the designations “in” and “out”, which had previously been used, can be ambiguous. Translocases are the most common secretion system in Gram positive bacteria.
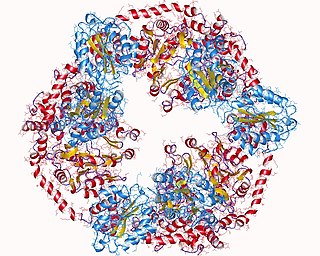
Magnesium-chelatase is a three-component enzyme (EC 6.6.1.1) that catalyses the insertion of Mg2+ into protoporphyrin IX. This is the first unique step in the synthesis of chlorophyll and bacteriochlorophyll. As a result, it is thought that Mg-chelatase has an important role in channeling intermediates into the (bacterio)chlorophyll branch in response to conditions suitable for photosynthetic growth:
In enzymology, a channel-conductance-controlling ATPase (EC 3.6.3.49) is an enzyme that catalyzes the chemical reaction
In enzymology, a chloroplast protein-transporting ATPase (EC 3.6.3.52) is an enzyme that catalyzes the chemical reaction
In enzymology, an iron-chelate-transporting ATPase (EC 3.6.3.34) is an enzyme that catalyzes the chemical reaction
In enzymology, a maltose-transporting ATPase (EC 3.6.3.19) is an enzyme that catalyzes the chemical reaction
In enzymology, a Mg2+-importing ATPase (EC 3.6.3.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a Na+-exporting ATPase (EC 3.6.3.7) is an enzyme that catalyzes the chemical reaction
In enzymology, a Na+-transporting two-sector ATPase (EC 3.6.3.15) is an enzyme that catalyzes the chemical reaction
In enzymology, an oligopeptide-transporting ATPase (EC 3.6.3.23) is an enzyme that catalyzes the chemical reaction
In enzymology, a phosphate-transporting ATPase (EC 3.6.3.27) is an enzyme that catalyzes the chemical reaction
In enzymology, a polar-amino-acid-transporting ATPase (EC 3.6.3.21) is an enzyme that catalyzes the chemical reaction
In enzymology, a proteasome ATPase (EC 3.6.4.8) is an enzyme that catalyzes the chemical reaction
In enzymology, a protein-secreting ATPase (EC 3.6.3.50) is an enzyme that catalyzes the chemical reaction

In enzymology, a vesicle-fusing ATPase (EC 3.6.4.6) is an enzyme that catalyzes the chemical reaction
In enzymology, a Zn2+-exporting ATPase (EC 3.6.3.5) is an enzyme that catalyzes the chemical reaction

The enzyme phosphatidate phosphatase (PAP, EC 3.1.3.4) is a key regulatory enzyme in lipid metabolism, catalyzing the conversion of phosphatidate to diacylglycerol:
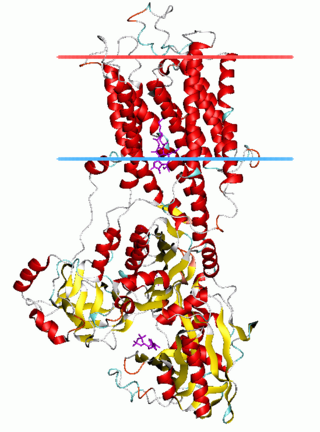
The P-type ATPases, also known as E1-E2 ATPases, are a large group of evolutionarily related ion and lipid pumps that are found in bacteria, archaea, and eukaryotes. P-type ATPases are α-helical bundle primary transporters named based upon their ability to catalyze auto- (or self-) phosphorylation (hence P) of a key conserved aspartate residue within the pump and their energy source, adenosine triphosphate (ATP). In addition, they all appear to interconvert between at least two different conformations, denoted by E1 and E2. P-type ATPases fall under the P-type ATPase (P-ATPase) Superfamily (TC# 3.A.3) which, as of early 2016, includes 20 different protein families.





