
N-ethylmaleimide-sensitive factor, also known as NSF or N-ethylmaleimide sensitive fusion proteins, is an enzyme which in humans is encoded by the NSF gene.
A kinesin is a protein belonging to a class of motor proteins found in eukaryotic cells. Kinesins move along microtubule (MT) filaments and are powered by the hydrolysis of adenosine triphosphate (ATP). The active movement of kinesins supports several cellular functions including mitosis, meiosis and transport of cellular cargo, such as in axonal transport, and intraflagellar transport. Most kinesins walk towards the plus end of a microtubule, which, in most cells, entails transporting cargo such as protein and membrane components from the center of the cell towards the periphery. This form of transport is known as anterograde transport. In contrast, dyneins are motor proteins that move toward the minus end of a microtubule in retrograde transport.

Molecular motors are natural (biological) or artificial molecular machines that are the essential agents of movement in living organisms. In general terms, a motor is a device that consumes energy in one form and converts it into motion or mechanical work; for example, many protein-based molecular motors harness the chemical free energy released by the hydrolysis of ATP in order to perform mechanical work. In terms of energetic efficiency, this type of motor can be superior to currently available man-made motors. One important difference between molecular motors and macroscopic motors is that molecular motors operate in the thermal bath, an environment in which the fluctuations due to thermal noise are significant.
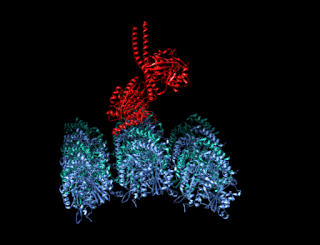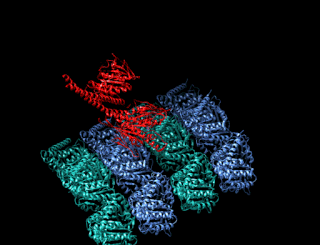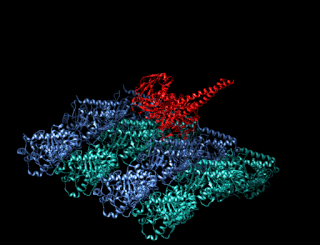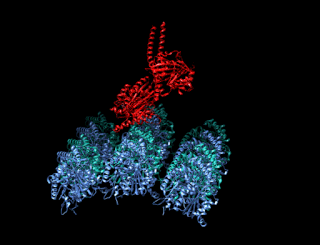
Minus-end-directed kinesin ATPase (EC 3.6.4.5) is an enzyme with systematic name kinesin ATP phosphohydrolase (minus-end-directed). This enzyme catalyses the following chemical reaction

26S protease regulatory subunit 6A, also known as 26S proteasome AAA-ATPase subunit Rpt5, is an enzyme that in humans is encoded by the PSMC3 gene. This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.

26S protease regulatory subunit 7, also known as 26S proteasome AAA-ATPase subunit Rpt1, is an enzyme that in humans is encoded by the PSMC2 gene This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex. Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.
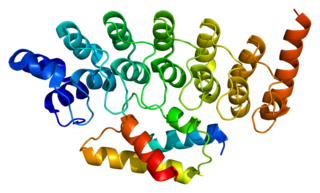
26S protease regulatory subunit 6B, also known as 26S proteasome AAA-ATPase subunit Rpt3, is an enzyme that in humans is encoded by the PSMC4 gene. This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.

26S protease regulatory subunit S10B, also known as 26S proteasome AAA-ATPase subunit Rpt4, is an enzyme that in humans is encoded by the PSMC6 gene. This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.

V-type proton ATPase subunit B, kidney isoform is an enzyme that in humans is encoded by the ATP6V1B1 gene.

Kinesin-like protein KIF23 is a protein that in humans is encoded by the KIF23 gene.

Kinesin-like protein KIF3A is a protein that in humans is encoded by the KIF3A gene.

V-type proton ATPase catalytic subunit A is an enzyme that in humans is encoded by the ATP6V1A gene.

V-type proton ATPase subunit G 1 is an enzyme that in humans is encoded by the ATP6V1G1 gene.

V-type proton ATPase subunit F is an enzyme that in humans is encoded by the ATP6V1F gene.

The ATP5MF gene encodes the ATP synthase subunit f, mitochondrial enzyme in humans.

V-type proton ATPase 21 kDa proteolipid subunit is an enzyme that in humans is encoded by the ATP6V0B gene.

Kinesin-like protein KIF3B is a protein that in humans is encoded by the KIF3B gene. KIF3B is an N-type protein that complexes with two other kinesin proteins to form two-headed anterograde motors. First, KIF3B forms a heterodimer with KIF3A ; (KIF3A/3B), that is membrane-bound and has ATPase activity. Then KIFAP3 binds to the tail domain to form a heterotrimeric motor. This motor has a plus end-directed microtubule sliding activity that exhibits a velocity of ~0.3 μm/s a. There are 14 kinesin protein families in the kinesin superfamily and KIF3B is part of the Kinesin-2 family, of kinesins that can all form heterotrimeric complexes. Expression of the three motor subunits is ubiquitous. The KIG3A/3B/KAP3 motors can transport 90 to 160 nm in diameter organelles.

Kinesin-like protein KIF14 is a protein that in humans is encoded by the KIF14 gene.

ATP synthase subunit O, mitochondrial is an enzyme that in humans is encoded by the ATP5PO gene.

Kinesin-like protein KIF11 is a molecular motor protein that is essential in mitosis. In humans it is coded for by the gene KIF11. Kinesin-like protein KIF11 is a member of the kinesin superfamily, which are nanomotors that move along microtubule tracks in the cell. Named from studies in the early days of discovery, it is also known as Kinesin-5, or as BimC, Eg5 or N-2, based on the founding members of this kinesin family.
















