
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in form of six loops of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Ligands can bind either to the extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists, although a spontaneous auto-activation of an empty receptor has also been observed.
GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved P-loop "G domain", a protein domain common to many GTPases.

G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases.
The signal recognition particle (SRP) is an abundant, cytosolic, universally conserved ribonucleoprotein that recognizes and targets specific proteins to the endoplasmic reticulum in eukaryotes and the plasma membrane in prokaryotes.
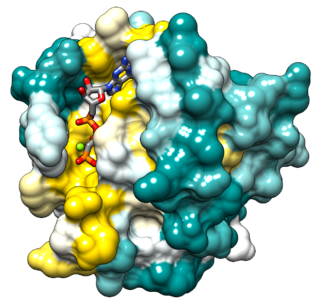
Ras, from "Rat sarcoma virus", is a family of related proteins that are expressed in all animal cell lineages and organs. All Ras protein family members belong to a class of protein called small GTPase, and are involved in transmitting signals within cells. Ras is the prototypical member of the Ras superfamily of proteins, which are all related in three-dimensional structure and regulate diverse cell behaviours.

Guanosine diphosphate, abbreviated GDP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside guanosine. GDP consists of a pyrophosphate group, a pentose sugar ribose, and the nucleobase guanine.

Alfred Goodman Gilman was an American pharmacologist and biochemist. He and Martin Rodbell shared the 1994 Nobel Prize in Physiology or Medicine "for their discovery of G-proteins and the role of these proteins in signal transduction in cells."
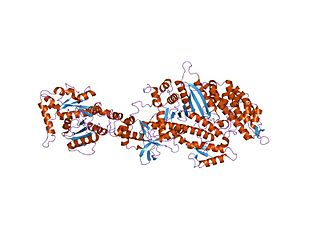
Dynamin is a GTPase responsible for endocytosis in the eukaryotic cell. Dynamin is part of the "dynamin superfamily", which includes classical dynamins, dynamin-like proteins, Mx proteins, OPA1, mitofusins, and GBPs. Members of the dynamin family are principally involved in the scission of newly formed vesicles from the membrane of one cellular compartment and their targeting to, and fusion with, another compartment, both at the cell surface as well as at the Golgi apparatus. Dynamin family members also play a role in many processes including division of organelles, cytokinesis and microbial pathogen resistance.

Ran also known as GTP-binding nuclear protein Ran is a protein that in humans is encoded by the RAN gene. Ran is a small 25 kDa protein that is involved in transport into and out of the cell nucleus during interphase and also involved in mitosis. It is a member of the Ras superfamily.

Signal recognition particle (SRP) receptor, also called the docking protein, is a dimer composed of 2 different subunits that are associated exclusively with the rough ER in mammalian cells. Its main function is to identify the SRP units. SRP is a molecule that helps the ribosome-mRNA-polypeptide complexes to settle down on the membrane of the endoplasmic reticulum.
In cell signalling, Son of Sevenless (SOS) refers to a set of genes encoding guanine nucleotide exchange factors that act on the Ras subfamily of small GTPases.

Guanine nucleotide exchange factors (GEFs) are proteins or protein domains that activate monomeric GTPases by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP). A variety of unrelated structural domains have been shown to exhibit guanine nucleotide exchange activity. Some GEFs can activate multiple GTPases while others are specific to a single GTPase.
Eukaryotic initiation factors (eIFs) are proteins or protein complexes involved in the initiation phase of eukaryotic translation. These proteins help stabilize the formation of ribosomal preinitiation complexes around the start codon and are an important input for post-transcription gene regulation. Several initiation factors form a complex with the small 40S ribosomal subunit and Met-tRNAiMet called the 43S preinitiation complex. Additional factors of the eIF4F complex recruit the 43S PIC to the five-prime cap structure of the mRNA, from which the 43S particle scans 5'-->3' along the mRNA to reach an AUG start codon. Recognition of the start codon by the Met-tRNAiMet promotes gated phosphate and eIF1 release to form the 48S preinitiation complex, followed by large 60S ribosomal subunit recruitment to form the 80S ribosome. There exist many more eukaryotic initiation factors than prokaryotic initiation factors, reflecting the greater biological complexity of eukaryotic translation. There are at least twelve eukaryotic initiation factors, composed of many more polypeptides, and these are described below.

Heterotrimeric G protein, also sometimes referred to as the "large" G proteins are membrane-associated G proteins that form a heterotrimeric complex. The biggest non-structural difference between heterotrimeric and monomeric G protein is that heterotrimeric proteins bind to their cell-surface receptors, called G protein-coupled receptors, directly. These G proteins are made up of alpha (α), beta (β) and gamma (γ) subunits. The alpha subunit is attached to either a GTP or GDP, which serves as an on-off switch for the activation of G-protein.

The signal recognition particle RNA, is part of the signal recognition particle (SRP) ribonucleoprotein complex. SRP recognizes the signal peptide and binds to the ribosome, halting protein synthesis. SRP-receptor is a protein that is embedded in a membrane, and which contains a transmembrane pore. When the SRP-ribosome complex binds to SRP-receptor, SRP releases the ribosome and drifts away. The ribosome resumes protein synthesis, but now the protein is moving through the SRP-receptor transmembrane pore.

Transforming protein RhoA, also known as Ras homolog family member A (RhoA), is a small GTPase protein in the Rho family of GTPases that in humans is encoded by the RHOA gene. While the effects of RhoA activity are not all well known, it is primarily associated with cytoskeleton regulation, mostly actin stress fibers formation and actomyosin contractility. It acts upon several effectors. Among them, ROCK1 and DIAPH1 are the best described. RhoA, and the other Rho GTPases, are part of a larger family of related proteins known as the Ras superfamily, a family of proteins involved in the regulation and timing of cell division. RhoA is one of the oldest Rho GTPases, with homologues present in the genomes since 1.5 billion years. As a consequence, RhoA is somehow involved in many cellular processes which emerged throughout evolution. RhoA specifically is regarded as a prominent regulatory factor in other functions such as the regulation of cytoskeletal dynamics, transcription, cell cycle progression and cell transformation.

Regulators of G protein signaling (RGS) are protein structural domains or the proteins that contain these domains, that function to activate the GTPase activity of heterotrimeric G-protein α-subunits.

Rho guanine nucleotide exchange factor 1 is a protein that in humans is encoded by the ARHGEF1 gene. This protein is also called RhoGEF1 or p115-RhoGEF.
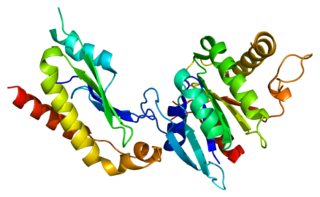
Signal recognition particle receptor subunit beta is a protein that in humans is encoded by the SRPRB gene.
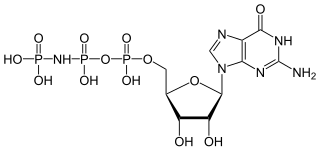
5'-Guanylyl imidodiphosphate (GDPNP) is a purine nucleotide. It is an analog of guanosine triphosphate in which one of the oxygen atoms is replaced with an amine, producing a non-hydrolyzable functional group. Guanylyl imidodiphosphate binds tightly to G-proteins in the presence of Mg2+. Guanylyl imidodiphosphate is a potent stimulator of adenylate cyclase. It is often used in studies of protein synthesis.
















