Trypsinogen is the precursor form of trypsin, a digestive enzyme. It is produced by the pancreas and found in pancreatic juice, along with amylase, lipase, and chymotrypsinogen. It is cleaved to its active form, trypsin, by enteropeptidase, which is found in the intestinal mucosa. Once activated, the trypsin can cleave more trypsinogen into trypsin, a process called autoactivation. Trypsin cleaves the peptide bond on the carboxyl side of basic amino acids such as arginine and lysine.
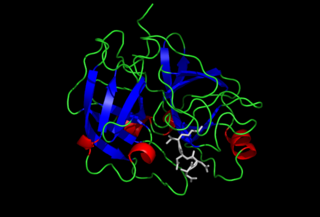
Enteropeptidase is an enzyme produced by cells of the duodenum and is involved in digestion in humans and other animals. Enteropeptidase converts trypsinogen into its active form trypsin, resulting in the subsequent activation of pancreatic digestive enzymes. Absence of enteropeptidase results in intestinal digestion impairment.

Hereditary pancreatitis (HP) is an inflammation of the pancreas due to genetic causes. It was first described in 1952 by Comfort and Steinberg but it was not until 1996 that Whitcomb et al isolated the first responsible mutation in the trypsinogen gene (PRSS1) on the long arm of chromosome seven (7q35).
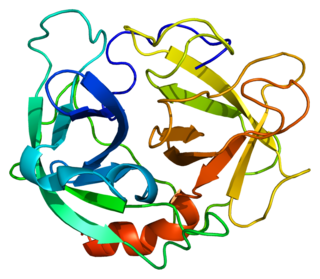
Neutrophil elastase is a serine proteinase in the same family as chymotrypsin and has broad substrate specificity. Neutrophil elastase is secreted by neutrophils during inflammation, and destroys bacteria and host tissue. It also localizes to neutrophil extracellular traps (NETs), via its high affinity for DNA, an unusual property for serine proteases.

Neutral and basic amino acid transport protein rBAT is a protein that in humans is encoded by the SLC3A1 gene.

Pancreatic secretory trypsin inhibitor (PSTI) also known as serine protease inhibitor Kazal-type 1 (SPINK1) or tumor-associated trypsin inhibitor (TATI) is a protein that in humans is encoded by the SPINK1 gene.

Suppressor of tumorigenicity 14 protein, also known as matriptase, is a protein that in humans is encoded by the ST14 gene. ST14 orthologs have been identified in most mammals for which complete genome data are available.

Glycophorin B (MNS blood group) (gene designation GYPB) also known as sialoglycoprotein delta and SS-active sialoglycoprotein is a protein which in humans is encoded by the GYPB gene. GYPB has also recently been designated CD235b (cluster of differentiation 235b).
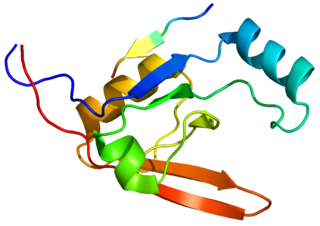
Serine protease HTRA1 is an enzyme that in humans is encoded by the HTRA1 gene. The HTRA1 protein is composed of four distinct protein domains. They are from amino-terminus to carboxyl-terminus an Insulin-like growth factor binding domain, a kazal domain, a trypsin-like peptidase domain and a PDZ domain.

Kallikrein-related peptidase 4 is a protein which in humans is encoded by the KLK4 gene.
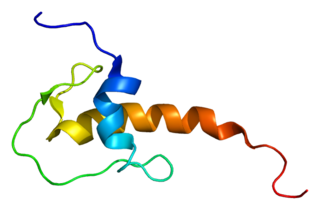
Lympho-epithelial Kazal-type-related inhibitor (LEKTI) also known as serine protease inhibitor Kazal-type 5 (SPINK5) is a protein that in humans is encoded by the SPINK5 gene.

Kallikrein-11 is a protein that in humans is encoded by the KLK11 gene.

Serpin B6 is a protein that in humans is encoded by the SERPINB6 gene.
Kallikrein-8 is a protein that in humans is encoded by the KLK8 gene.

Chymotrypsin-like elastase family member 3B also known as elastase-3B, protease E, or fecal elastase is an enzyme that in humans is encoded by the CELA3B gene.

Chymotrypsin-like elastase family member 3A is an enzyme that in humans is encoded by the CELA3A gene.

Chymotrypsin C, also known as caldecrin or elastase 4, is an enzyme that in humans is encoded by the CTRC gene.

Brain-specific serine protease 4 (BSSP-4), also known as serine protease 22 or tryptase epsilon, is an enzyme that in humans is encoded by the PRSS22 gene.

Protease, serine, 2 is a protein that in humans is encoded by the PRSS2 gene.

Protease, serine, 3 is a protein that in humans is encoded by the PRSS3 gene.























