
Glycogen phosphorylase is one of the phosphorylase enzymes. Glycogen phosphorylase catalyzes the rate-limiting step in glycogenolysis in animals by releasing glucose-1-phosphate from the terminal alpha-1,4-glycosidic bond. Glycogen phosphorylase is also studied as a model protein regulated by both reversible phosphorylation and allosteric effects.
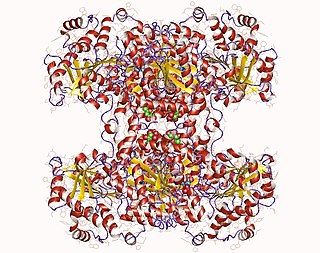
Glycogen synthase is a key enzyme in glycogenesis, the conversion of glucose into glycogen. It is a glycosyltransferase that catalyses the reaction of UDP-glucose and n to yield UDP and n+1.

A debranching enzyme is a molecule that helps facilitate the breakdown of glycogen, which serves as a store of glucose in the body, through glucosyltransferase and glucosidase activity. Together with phosphorylases, debranching enzymes mobilize glucose reserves from glycogen deposits in the muscles and liver. This constitutes a major source of energy reserves in most organisms. Glycogen breakdown is highly regulated in the body, especially in the liver, by various hormones including insulin and glucagon, to maintain a homeostatic balance of blood-glucose levels. When glycogen breakdown is compromised by mutations in the glycogen debranching enzyme, metabolic diseases such as Glycogen storage disease type III can result.

Purine nucleoside phosphorylase, PNP, PNPase or inosine phosphorylase is an enzyme that in humans is encoded by the NP gene. It catalyzes the chemical reaction

Tyrosine-protein phosphatase non-receptor type 11 (PTPN11) also known as protein-tyrosine phosphatase 1D (PTP-1D), Src homology region 2 domain-containing phosphatase-2 (SHP-2), or protein-tyrosine phosphatase 2C (PTP-2C) is an enzyme that in humans is encoded by the PTPN11 gene. PTPN11 is a protein tyrosine phosphatase (PTP) Shp2.

Phosphorylase kinase (PhK) is a serine/threonine-specific protein kinase which activates glycogen phosphorylase to release glucose-1-phosphate from glycogen. PhK phosphorylates glycogen phosphorylase at two serine residues, triggering a conformational shift which favors the more active glycogen phosphorylase “a” form over the less active glycogen phosphorylase b.

Myophosphorylase or glycogen phosphorylase, muscle associated (PYGM) is the muscle isoform of the enzyme glycogen phosphorylase and is encoded by the PYGM gene. This enzyme helps break down glycogen into glucose-1-phosphate, so it can be used within the muscle cell. Mutations in this gene are associated with McArdle disease, a glycogen storage disease of muscle.
Glucose-1,6-bisphosphate synthase is a type of enzyme called a phosphotransferase and is involved in mammalian starch and sucrose metabolism. It catalyzes the transfer of a phosphate group from 1,3-bisphosphoglycerate to glucose-1-phosphate, yielding 3-phosphoglycerate and glucose-1,6-bisphosphate.

Serine/threonine-protein phosphatase PP1-alpha catalytic subunit is an enzyme that in humans is encoded by the PPP1CA gene.

Serine/threonine-protein phosphatase 2A 65 kDa regulatory subunit A alpha isoform is an enzyme that in humans is encoded by the PPP2R1A gene. In the plant Arabidopsis thaliana a similar enzyme is encoded by the RCN1 gene (At1g25490).

Serine/threonine-protein phosphatase PP1-gamma catalytic subunit is an enzyme that in humans is encoded by the PPP1CC gene.

Myosin light-chain phosphatase, also called myosin phosphatase (EC 3.1.3.53; systematic name [myosin-light-chain]-phosphate phosphohydrolase), is an enzyme (specifically a serine/threonine-specific protein phosphatase) that dephosphorylates the regulatory light chain of myosin II:

The enzyme phosphatidate phosphatase (PAP, EC 3.1.3.4) is a key regulatory enzyme in lipid metabolism, catalyzing the conversion of phosphatidate to diacylglycerol:

Serine/threonine-protein phosphatase 2A regulatory subunit B'' subunit alpha is an enzyme that in humans is encoded by the PPP2R3A gene. Protein phosphatase 2 is one of the four major Ser/Thr phosphatases and is implicated in the negative control of cell growth and division. Protein phosphatase 2 holoenzymes are heterotrimeric proteins composed of a structural subunit A, a catalytic subunit C, and a regulatory subunit B. The regulatory subunit is encoded by a diverse set of genes that have been grouped into the B/PR55, B'/PR61, and B''/PR72 families. These different regulatory subunits confer distinct enzymatic specificities and intracellular localizations to the holozenzyme. The product of this gene belongs to the B'' family. The B'' family has been further divided into subfamilies. The product of this gene belongs to the alpha subfamily of regulatory subunit B''. Alternative splicing results in multiple transcript variants encoding different isoforms.

Phosphorylase b kinase gamma catalytic chain, skeletal muscle isoform is an enzyme that in humans is encoded by the PHKG1 gene.

Protein tyrosine phosphatase type IVA 1 is an enzyme that in humans is encoded by the PTP4A1 gene.

Protein phosphorylation is a reversible post-translational modification of proteins in which an amino acid residue is phosphorylated by a protein kinase by the addition of a covalently bound phosphate group. Phosphorylation alters the structural conformation of a protein, causing it to become either activated or deactivated, or otherwise modifying its function. Approximately 13000 human proteins have sites that are phosphorylated.

Protein phosphatase 1 regulatory subunit 12B is an enzyme that in humans is encoded by the PPP1R12B gene.

Phosphorylase b kinase regulatory subunit alpha, skeletal muscle isoform is an enzyme that in humans is encoded by the PHKA1 gene. It is the muscle isoform of Phosphorylase kinase (PhK).

Protein phosphatase 1 (PP1) belongs to a certain class of phosphatases known as protein serine/threonine phosphatases. This type of phosphatase includes metal-dependent protein phosphatases (PPMs) and aspartate-based phosphatases. PP1 has been found to be important in the control of glycogen metabolism, muscle contraction, cell progression, neuronal activities, splicing of RNA, mitosis, cell division, apoptosis, protein synthesis, and regulation of membrane receptors and channels.



















