
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula CH3OH (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, volatile, colorless and flammable liquid with a distinctive alcoholic odour similar to that of ethanol (potable alcohol). Methanol acquired the name wood alcohol because it was once produced chiefly by the destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide.
Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. The reaction can also be accomplished with the help of other enzymes, particularly lipases.
Biodiesel production is the process of producing the biofuel, biodiesel, through the chemical reactions of transesterification and esterification. This involves vegetable or animal fats and oils being reacted with short-chain alcohols. The alcohols used should be of low molecular weight. Ethanol is the most used because of its low cost, however, greater conversions into biodiesel can be reached using methanol. Although the transesterification reaction can be catalyzed by either acids or bases, the base-catalyzed reaction is more common. This path has lower reaction times and catalyst cost than those acid catalysis. However, alkaline catalysis has the disadvantage of high sensitivity to both water and free fatty acids present in the oils.August 10th is international biodiesel day

Methyl acetate, also known as MeOAc, acetic acid methyl ester or methyl ethanoate, is a carboxylate ester with the formula CH3COOCH3. It is a flammable liquid with a characteristically pleasant smell reminiscent of some glues and nail polish removers. Methyl acetate is occasionally used as a solvent, being weakly polar and lipophilic, but its close relative ethyl acetate is a more common solvent being less toxic and less soluble in water. Methyl acetate has a solubility of 25% in water at room temperature. At elevated temperature its solubility in water is much higher. Methyl acetate is not stable in the presence of strong aqueous bases or aqueous acids. Methyl acetate is not considered a VOC in the USA.

Cocaethylene (ethylbenzoylecgonine) is the ethyl ester of benzoylecgonine. It is structurally similar to cocaine, which is the methyl ester of benzoylecgonine. Cocaethylene is formed by the liver when cocaine and ethanol coexist in the blood. In 1885, cocaethylene was first synthesized, and in 1979, cocaethylene's side effects were discovered.
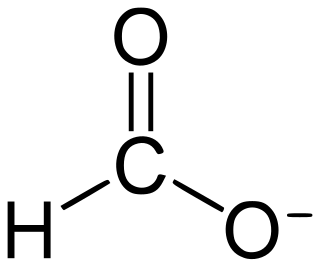
Formate is the conjugate base of formic acid. Formate is an anion or its derivatives such as ester of formic acid. The salts and esters are generally colorless.

Dimethyl terephthalate (DMT) is an organic compound with the formula C6H4(COOCH3)2. It is the diester formed from terephthalic acid and methanol. It is a white solid that melts to give a distillable colourless liquid.

Phosphoenolpyruvate is the ester derived from the enol of pyruvate and phosphate. It exists as an anion. PEP is an important intermediate in biochemistry. It has the highest-energy phosphate bond found in organisms, and is involved in glycolysis and gluconeogenesis. In plants, it is also involved in the biosynthesis of various aromatic compounds, and in carbon fixation; in bacteria, it is also used as the source of energy for the phosphotransferase system.

Methyl benzoate is an organic compound. It is an ester with the chemical formula C6H5CO2CH3. It is a colorless liquid that is poorly soluble in water, but miscible with organic solvents. Methyl benzoate has a pleasant smell, strongly reminiscent of the fruit of the feijoa tree, and it is used in perfumery. It also finds use as a solvent and as a pesticide used to attract insects such as orchid bees.

In organic chemistry, an ortho ester is a functional group containing three alkoxy groups attached to one carbon atom, i.e. with the general formula RC(OR′)3. Orthoesters may be considered as products of exhaustive alkylation of unstable orthocarboxylic acids and it is from these that the name 'ortho ester' is derived. An example is ethyl orthoacetate, CH3C(OCH2CH3)3, more correctly known as 1,1,1-triethoxyethane.
Pectinesterase (EC 3.1.1.11; systematic name pectin pectylhydrolase) is a ubiquitous cell-wall-associated enzyme that presents several isoforms that facilitate plant cell wall modification and subsequent breakdown. It catalyzes the following reaction:
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is converted into fatty acids is derived from carbohydrates via the glycolytic pathway. The glycolytic pathway also provides the glycerol with which three fatty acids can combine to form triglycerides, the final product of the lipogenic process. When only two fatty acids combine with glycerol and the third alcohol group is phosphorylated with a group such as phosphatidylcholine, a phospholipid is formed. Phospholipids form the bulk of the lipid bilayers that make up cell membranes and surrounds the organelles within the cells.

Methyl acrylate is an organic compound, more accurately the methyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced to make acrylate fiber, which is used to weave synthetic carpets. It is also a reagent in the synthesis of various pharmaceutical intermediates. Owing to the tendency of methyl acrylate to polymerize, samples typically contain an inhibitor such as hydroquinone.
In enzymology, a methylmalonyl-CoA carboxytransferase is an enzyme that catalyzes the chemical reaction

The enzyme protein-glutamate methylesterase (EC 3.1.1.61) catalyzes the reaction

Macrophomic acid is a fungal metabolite isolated from the fungus Macrophoma commelinae. The enzyme macrophomate synthase converts 5-acetyl-4-methoxy-6-methyl-2-pyrone to 4-acetyl-3-methoxy-5-methyl-benzoic acid through an unusual intermolecular Diels-Alder reaction. The pathway to formation of macrophomic acid suggests that the enzyme is a natural Diels-Alderase. Formation of this type of aromatic ring compound normally proceeds via the shikimate and polyketide pathways; however, the production of macrophomic acid by macrophomate synthase proceeds totally differently. Learning about the production of macrophomic acid by a possible natural Diels-Alderase enzyme is important in understanding enzyme catalytic mechanisms. This knowledge can then be applied to organic synthesis.

Trimethyl orthoformate (TMOF) is the organic compound with the formula HC(OCH3)3. A colorless liquid, it is the simplest orthoester. It is a reagent used in organic synthesis for the formation of methyl ethers. The product of reaction of an aldehyde with trimethyl orthoformate is an acetal. In general cases, these acetals can be deprotected back to the aldehyde by using hydrochloric acid.
The enzyme Pimelyl-[acyl-carrier protein] methyl ester esterase (EC 3.1.1.85, BioH; systematic name pimelyl-[acyl-carrier protein] methyl ester hydrolase catalyses the reaction
The enzyme protein phosphatase methylesterase-1 (EC 3.1.1.89, PME-1, PPME1; systematic name (phosphatase 2A protein)-leucine ester acylhydrolase catalyses the reaction

C1 chemistry is the chemistry of one-carbon molecules. Although many compounds and ions contain only one carbon, stable and abundant C-1 feedstocks are the focus of research. Four compounds are of major industrial importance: methane, carbon monoxide, carbon dioxide, and methanol. Technologies that interconvert these species are often used massively to match supply to demand.















