
Benzoic acid is a white solid organic compound with the formula C6H5COOH, whose structure consists of a benzene ring with a carboxyl substituent. The benzoyl group is often abbreviated "Bz", thus benzoic acid is also denoted as BzOH, since the benzoyl group has the formula –C6H5CO. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source.

A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.

Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's diethyl ester. The name originates from the Greek word μᾶλον (malon) meaning 'apple'.

Benzyl alcohol (also known as α-Cresol) is an aromatic alcohol with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful as a solvent for its polarity, low toxicity, and low vapor pressure. Benzyl alcohol has moderate solubility in water (4 g/100 mL) and is miscible in alcohols and diethyl ether. The anion produced by deprotonation of the alcohol group is known as benzylate or benzyloxide.
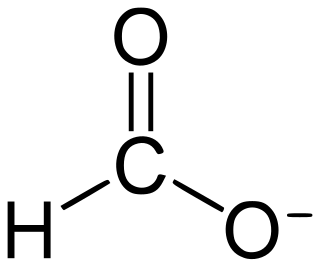
Formate is the conjugate base of formic acid. Formate is an anion or its derivatives such as ester of formic acid. The salts and esters are generally colorless.

Benzyl chloroformate, also known as benzyl chlorocarbonate or Z-chloride, is the benzyl ester of chloroformic acid. It can be also described as the chloride of the benzyloxycarbonyl group. In its pure form it is a water-sensitive oily colorless liquid, although impure samples usually appear yellow. It possesses a characteristic pungent odor and degrades in contact with water.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).

Benzyl butyl phthalate (BBP) is an organic compound historically used a plasticizer, but which has now been largely phased out due to health concerns. It is a phthalate ester of containing benzyl alcohol, and n-butanol tail groups. Like most phthalates, BBP is non-volatile and remains liquid over a wide range of temperatures. It was mostly used as a plasticizer for PVC, but was also a common plasticizer for PVCA and PVB.

Benzyl acetate is an organic ester with the molecular formula CH3C(O)OCH2C6H5. It is formed by the condensation of benzyl alcohol and acetic acid.

Benzyl benzoate is an organic compound which is used as a medication and insect repellent. As a medication it is used to treat scabies and lice. For scabies either permethrin or malathion is typically preferred. It is applied to the skin as a lotion. Typically two to three applications are needed. It is also present in Balsam of Peru, Tolu balsam, and in a number of flowers.
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.
The Gabriel–Colman rearrangement is the chemical reaction of a saccharin or phthalimido ester with a strong base, such as an alkoxide, to form substituted isoquinolines. First described in 1900 by chemists Siegmund Gabriel and James Colman, this rearrangement, a ring expansion, is seen to be general if there is an enolizable hydrogen on the group attached to the nitrogen, since it is necessary for the nitrogen to abstract a hydrogen to form the carbanion that will close the ring. As shown in the case of the general example below, X is either CO or SO2.

In organic chemistry, benzoyl is the functional group with the formula −COC6H5 and structure −C(=O)−C6H5. It can be viewed as benzaldehyde missing one hydrogen. The benzoyl group has a mass of 105 amu.
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula H2NCOOH. It can be obtained by the reaction of ammonia NH3 and carbon dioxide CO2 at very low temperatures, which also yields ammonium carbamate [NH4]+[NH2CO2]−. The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases. The solid apparently consists of dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.

The Erlenmeyer–Plöchl azlactone and amino acid synthesis, named after Friedrich Gustav Carl Emil Erlenmeyer who partly discovered the reaction, is a series of chemical reactions which transform an N-acyl glycine to various other amino acids via an oxazolone.
In enzymology, a thiocyanate isomerase is an enzyme that catalyzes the chemical reaction

The Mukaiyama taxol total synthesis published by the group of Teruaki Mukaiyama of the Tokyo University of Science between 1997 and 1999 was the 6th successful taxol total synthesis. The total synthesis of Taxol is considered a hallmark in organic synthesis.

Benzyl salicylate is a salicylic acid benzyl ester, a chemical compound most frequently used in cosmetics as a fragrance additive or UV light absorber. It appears as an almost colorless liquid with a mild odor described as "very faint, sweet-floral, slightly balsamic" by some, while others smell nothing at all. There is debate whether the odour is caused solely by impurities or a genetic predisposition. It occurs naturally in a variety of plants and plant extracts and is widely used in blends of fragrance materials.

Tetrachlorvinphos is an organophosphate insecticide used to kill fleas and ticks.
Benzyl alcohol O-benzoyltransferase is an enzyme with systematic name benzoyl-CoA:benzyl alcohol O-benzoyltransferase. This enzyme catalyses the following chemical reaction















