
Norbuprenorphine is a major active metabolite of the opioid modulator buprenorphine. It is a μ-opioid, δ-opioid, and nociceptin receptor full agonist, and a κ-opioid receptor partial agonist. In rats, unlike buprenorphine, norbuprenorphine produces marked respiratory depression but with very little antinociceptive effect. In explanation of these properties, norbuprenorphine has been found to be a high affinity P-glycoprotein substrate, and in accordance, shows very limited blood-brain-barrier penetration.
Pravadoline (WIN 48,098) is an antinflammatory and analgesic drug with an IC50 of 4.9 μM and a Ki of 2511 nM at CB1, related in structure to nonsteroidal anti-inflammatory drugs (NSAIDs) such as indometacin. It was developed in the 1980s as a new antiinflammatory and prostaglandin synthesis inhibitor, acting through inhibition of the enzyme cyclooxygenase (COX).

DPI-3290 was discovered by scientists at Burroughs Wellcome and licensed to Delta Pharmaceutical and is a drug that is used in scientific research. It is a potent analgesic drug, which produces little respiratory depression.

Tebanicline is a potent synthetic nicotinic (non-opioid) analgesic drug developed by Abbott. It was developed as a less toxic analog of the potent poison dart frog-derived compound epibatidine, which is about 200 times stronger than morphine as an analgesic, but produces extremely dangerous toxic side effects. Like epibatidine, tebanicline showed potent analgesic activity against neuropathic pain in both animal and human trials, but with far less toxicity than its parent compound. It acts as a partial agonist at neuronal nicotinic acetylcholine receptors, binding to both the α3β4 and the α4β2 subtypes.
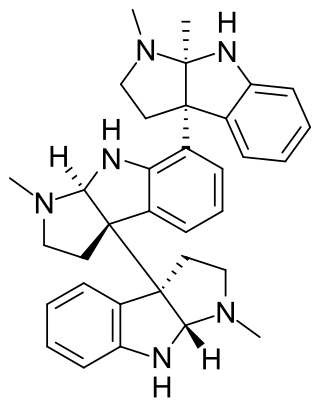
Hodgkinsine is an alkaloid found in plants of the genus Psychotria, particularly Psychotria colorata, although it is also found in Psychotria lyciiflora and probably other species in this family,

Glaucine(1,2,9,10-TetraMethoxyAporphine, Bromcholitin, Glauvent, Tusidil, Tussiglaucin) is an aporphine alkaloid found in several different plant species in the family Papaveraceae such as Glaucium flavum, Glaucium oxylobum and Corydalis yanhusuo, and in other plants like Croton lechleri in the family Euphorbiaceae.

Dimethylphenylpiperazinium (DMPP) is a nicotinic acetylcholine receptor agonist which is selective for the ganglionic subtype. One of the earliest reports on the pharmacology of DMPP, describing it as a ganglion-stimulating, hypertensive agent, came from Graham Chen and his co-workers at Parke, Davis & Co.

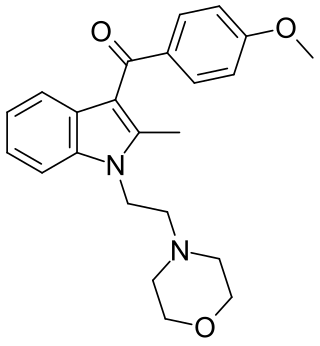
Vedaclidine (INN, codenamed LY-297,802, NNC 11-1053) is an experimental analgesic drug which acts as a mixed agonist–antagonist at muscarinic acetylcholine receptors, being a potent and selective agonist for the M1 and M4 subtypes, yet an antagonist at the M2, M3 and M5 subtypes. It is orally active and an effective analgesic over 3× the potency of morphine, with side effects such as salivation and tremor only occurring at many times the effective analgesic dose. Human trials showed little potential for development of dependence or abuse, and research is continuing into possible clinical application in the treatment of neuropathic pain and cancer pain relief.
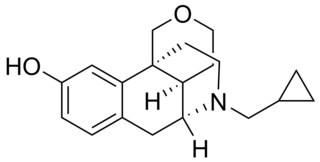
Proxorphan (INN), also known as proxorphan tartate (USAN), is an opioid analgesic and antitussive drug of the morphinan family that was never marketed. It acts preferentially as a κ-opioid receptor partial agonist and to a lesser extent as a μ-opioid receptor partial agonist.
The hot plate test is a test of the pain response in animals, similar to the tail flick test. Both hot plate and tail-flick methods are used generally for centrally acting analgesic, while peripherally acting drugs are ineffective in these tests but sensitive to acetic acid-induced writhing test.

1-Methylamino-1-(3,4-methylenedioxyphenyl)propane or M-ALPHA is an empathogen, reported by Alexander Shulgin in his book PIHKAL as a positional isomer of MDMA, and subsequently found being sold as a designer drug in the UK in 2010, and reported to the EMCDDA new drug monitoring service. It was described by Alexander Shulgin as similar in action to its demethylated homologue, ALPHA, but with roughly twice the duration and twice the potency.

Kelatorphan is a drug which acts as a powerful and complete inhibitor of nearly all of the enzymes responsible for catabolism of the endogenous enkephalins, including neutral endopeptidase (NEP), dipeptidyl peptidase III (DPP3), aminopeptidase N (APN), and angiotensin-converting enzyme (ACE). In mice, with the intracerebroventricular co-administration of a 50 µg dose of kelatorphan (this route is necessary because kelatorphan is incapable of crossing the blood-brain-barrier) hence alongside exogenous [Met]enkephalin (ED50 approximately 10 ng), it potentiated the analgesic effects of the latter by 50,000 times. Kelatorphan also displays potent antinociceptive effects alone, and does not depress respiration, although at high doses it actually increases it.
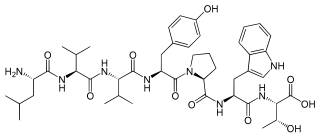
Spinorphin is an endogenous, non-classical opioid peptide of the hemorphin family first isolated from the bovine spinal cord (hence the prefix spin-) and acts as a regulator of the enkephalinases, a class of enzymes that break down endogenous the enkephalin peptides. It does so by inhibiting the enzymes aminopeptidase N (APN), dipeptidyl peptidase III (DPP3), angiotensin-converting enzyme (ACE), and neutral endopeptidase (NEP). Spinorphin is a heptapeptide and has the amino acid sequence Leu-Val-Val-Tyr-Pro-Trp-Thr (LVVYPWT). It has been observed to possess antinociceptive, antiallodynic, and anti-inflammatory properties. The mechanism of action of spinorphin has not been fully elucidated (i.e., how it acts to inhibit the enkephalinases), but it has been found to act as an antagonist of the P2X3 receptor, and as a weak partial agonist/antagonist of the FP1 receptor.

Hemorphin-4 is an endogenous opioid peptide of the hemorphin family which possesses antinociceptive properties and is derived from the β-chain of hemoglobin in the bloodstream. It is a tetrapeptide with the amino acid sequence Tyr-Pro-Trp-Thr. Hemorphin-4 has affinities for the μ-, δ-, and κ-opioid receptors that are in the same range as the structurally related β-casomorphins, although affinity to the κ-opioid receptor is markedly higher in comparison. It acts as an agonist at these sites. Hemorphin-4 also has inhibitory effects on angiotensin-converting enzyme (ACE), and as a result, may play a role in the regulation of blood pressure. Notably, inhibition of ACE also reduces enkephalin catabolism.
Hemorphins are a class of naturally occurring, endogenous opioid peptides which are found in the bloodstream, and are derived from the β-chain of hemoglobin. They have antinociceptive effects via activation of the opioid receptors, and some may also play a role in blood pressure through inhibition of the angiotensin-converting enzyme (ACE), as well as cause an elevation of endogenous enkephalin levels. Some examples of hemorphins include hemorphin-4, spinorphin, and valorphin.

Biphalin is a dimeric enkephalin endogenous peptide (Tyr-D-Ala-Gly-Phe-NH)2 composed of two tetrapeptides derived from enkephalins, connected 'tail-to-tail' by a hydrazide bridge. The presence of two distinct pharmacophores confers on biphalin a high affinity for both μ and δ opioid receptors (with an EC50 of about 1-5 nM for both μ and δ receptors), therefore it has analgesic activity. Biphalin presents a considerable antinociceptive profile. In fact, when administered intracerebroventricularly in mice, biphalin displays a potency almost 7-fold greater than that of the ultra-potent alkaloid agonist, etorphine and 7000-fold greater than morphine; biphalin and morphine were found to be equipotent after intraperitoneal administration. The extraordinary in vivo potency shown by this compound is coupled with low side-effects, in particular, to produce no dependency in chronic use. For these reasons, several efforts have been carried out in order to obtain more information about structure-activity relationship (SAR). Results clearly indicate that, at least for μ receptor binding, the presence of two pharmacophores is not necessary; Tyr1 is indispensable for analgesic activity, while replacing Phe at the position 4 and 4' with non-aromatic, but lipophilic amino acids does not greatly change the binding properties and in general 4,4' positions are found to be important to design biphalin analogues with increased potency and modified μ/δ selectivity. The hydrazide linker is not fundamental for activity or binding, and it can be conveniently substituted by different conformationally constrained cycloaliphatic diamine linkers.

LY-235959 is a competitive antagonist at the NMDA receptor. It has analgesic and neuroprotective effects and causes hypothermia in animal models, as well as reducing the development of tolerance to morphine and altering the reinforcing effects of cocaine.
PF-3845 is a selective inhibitor of fatty acid amide hydrolase. It results in increased levels of anandamide and results in cannabinoid receptor-based effects. It has anti-inflammatory action in mice colitis models. Antidiarrheal and antinociceptive effects were also seen in mouse models of pain.

Opiranserin (INN; developmental code name VVZ-149) is a selective and combined glycine GlyT2 transporter blocker (IC50 = 0.86 μM), purine P2X3 receptor antagonist (IC50 = 0.87 μM), and serotonin 5-HT2A receptor antagonist (IC50 = 1.3 μM) which is under development by Vivozon for the intravenous treatment of postoperative pain. As of April 2017, it is in phase II clinical trials for this indication. The INN of the drug was issued in 2017.

6-Chloronicotine is a drug which acts as an agonist at neural nicotinic acetylcholine receptors. It substitutes for nicotine in animal studies with around twice the potency, and shows antinociceptive effects.

















