Structural studies

As of late 2007, 3 structures have been solved for this class of enzymes, with PDB accession codes 1TBU, 2Q2B, and 2QQ2.
| palmitoyl-CoA hydrolase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 3.1.2.2 | ||||||||
| CAS no. | 9025-87-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Palmitoyl-CoA hydrolase (EC 3.1.2.2) is an enzyme in the family of hydrolases that specifically acts on thioester bonds. It catalyzes the hydrolysis of long chain fatty acyl thioesters of acyl carrier protein or coenzyme A to form free fatty acid and the corresponding thiol:
It has a strict specificity for thioesters with a chain link greater than C10.
These enzymes are localized in almost all cellular compartments, such as endoplasmic reticulum, cytosol, mitochondria, and peroxisomes. They are highly regulated by peroxisome proliferator activated receptors, which led to their involvement in lipid metabolism. The enzyme is up-regulated during times of increased fatty acid oxidation, which suggests that this enzyme has a potential role the peroxisomal beta-oxidation.
The systematic name is palmitoyl-CoA hydrolase. Other names in common use include long-chain fatty-acyl-CoA hydrolase, palmitoyl coenzyme A hydrolase, palmitoyl thioesterase, palmitoyl coenzyme A hydrolase, palmitoyl-CoA deacylase, palmityl thioesterase, palmityl-CoA deacylase, fatty acyl thioesterase I, and palmityl thioesterase I.

As of late 2007, 3 structures have been solved for this class of enzymes, with PDB accession codes 1TBU, 2Q2B, and 2QQ2.
At a subcellular level, palmitoyl-CoA hydrolase is localized in the endoplasmic reticulum, cytosol, mitochondria, and peroxisomes. Studies have shown that in rats that are fed high fat diets, palmitoyl-CoA hydrolase activity in the liver increased. While the details of the mechanism are not known, the results suggest that there is an "induction" mechanism taking place for palmitoyl-CoA hydrolase and peroxisomal beta-oxidation enzymes.
Diabetes is the most common cause of liver disease in the U.S., type 2 diabetes. Studies have been done to show that, while there is not direct correlation between palmitoyl-CoA hydrolase and diabetes, streptozotocin-induced diabetes significantly decreased rat liver palmitoyl-CoA hydrolase. This led to high levels of fatty acyl-CoA being present in the liver, which shows that a diseased liver cannot regulate the amount of fatty acyl-CoA that is present versus a normal, healthy liver. A defect in acyl-CoA degradation in livers can produce hyperammonemia and hypoglycemia.
Enoyl-CoA-(∆) isomerase (EC 5.3.3.8, also known as dodecenoyl-CoA- isomerase, 3,2-trans-enoyl-CoA isomerase, ∆3 ,∆2 -enoyl-CoA isomerase, or acetylene-allene isomerase, is an enzyme that catalyzes the conversion of cis- or trans-double bonds of coenzyme A bound fatty acids at gamma-carbon to trans double bonds at beta-carbon as below:
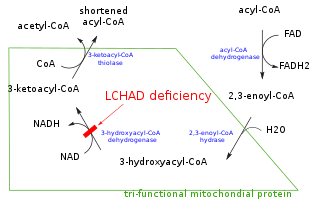
In biochemistry and metabolism, beta oxidation (also β-oxidation) is the catabolic process by which fatty acid molecules are broken down in the cytosol in prokaryotes and in the mitochondria in eukaryotes to generate acetyl-CoA, which enters the citric acid cycle, and NADH and FADH2, which are co-enzymes used in the electron transport chain. It is named as such because the beta carbon of the fatty acid undergoes oxidation to a carbonyl group. Beta-oxidation is primarily facilitated by the mitochondrial trifunctional protein, an enzyme complex associated with the inner mitochondrial membrane, although very long chain fatty acids are oxidized in peroxisomes.
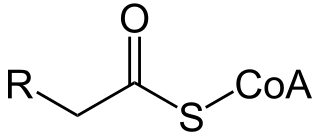
Acyl-CoA is a group of coenzymes that metabolize fatty acids. Acyl-CoA's are susceptible to beta oxidation, forming, ultimately, acetyl-CoA. The acetyl-CoA enters the citric acid cycle, eventually forming several equivalents of ATP. In this way, fats are converted to ATP, the universal biochemical energy carrier.

Thiolases, also known as acetyl-coenzyme A acetyltransferases (ACAT), are enzymes which convert two units of acetyl-CoA to acetoacetyl CoA in the mevalonate pathway.
The enzyme acyl-CoA hydrolase (EC 3.1.2.20) catalyzes the reaction
The enzyme ADP-dependent short-chain-acyl-CoA hydrolase (EC 3.1.2.18) catalyzes the reaction
The enzyme choloyl-CoA hydrolase (EC 3.1.2.27) catalyzes the reaction

Palmitoyl protein hydrolase/thioesterases is an enzyme (EC 3.1.2.22) that removes thioester-linked fatty acyl groups such as palmitate from modified cysteine residues in proteins or peptides during lysosomal degradation. It catalyzes the reaction

Peroxisomal acyl-coenzyme A oxidase 1 is an enzyme that in humans is encoded by the ACOX1 gene.

Long-chain-fatty-acid—CoA ligase 1 is an enzyme that in humans is encoded by the ACSL1 gene.

Acyl-coenzyme A thioesterase 8 is an enzyme that in humans is encoded by the ACOT8 gene.

Acyl-CoA thioesterase 2, also known as ACOT2, is an enzyme which in humans is encoded by the ACOT2 gene.

Cytosolic acyl coenzyme A thioester hydrolase is an enzyme that in humans is encoded by the ACOT7 gene.

Acyl-coenzyme A thioesterase 4 is an enzyme that in humans is encoded by the ACOT4 gene.

Acyl-coenzyme A thioesterase 11 also known as StAR-related lipid transfer protein 14 (STARD14) is an enzyme that in humans is encoded by the ACOT11 gene. This gene encodes a protein with acyl-CoA thioesterase activity towards medium (C12) and long-chain (C18) fatty acyl-CoA substrates which relies on its StAR-related lipid transfer domain. Expression of a similar murine protein in brown adipose tissue is induced by cold exposure and repressed by warmth. Expression of the mouse protein has been associated with obesity, with higher expression found in obesity-resistant mice compared with obesity-prone mice. Alternative splicing results in two transcript variants encoding different isoforms.

Acyl-CoA thioesterase 6 is a protein that in humans is encoded by the ACOT6 gene. The protein, also known as C14orf42, is an enzyme with thioesterase activity.
Long-chain acyl-CoA dehydrogenase is an enzyme with systematic name long-chain acyl-CoA:electron-transfer flavoprotein 2,3-oxidoreductase. This enzyme catalyses the following chemical reaction

Acyl-CoA thioesterase 9 is a protein that is encoded by the human ACOT9 gene. It is a member of the acyl-CoA thioesterase superfamily, which is a group of enzymes that hydrolyze Coenzyme A esters. There is no known function, however it has been shown to act as a long-chain thioesterase at low concentrations, and a short-chain thioesterase at high concentrations.

Acyl-CoA thioesterase 13 is a protein that in humans is encoded by the ACOT13 gene. This gene encodes a member of the thioesterase superfamily. In humans, the protein co-localizes with microtubules and is essential for sustained cell proliferation.

Acyl-CoA thioesterase 1 is a protein that in humans is encoded by the ACOT1 gene.