Hydrolase is a class of enzymes that commonly perform as biochemical catalysts that use water to break a chemical bond, which typically results in dividing a larger molecule into smaller molecules. Some common examples of hydrolase enzymes are esterases including lipases, phosphatases, glycosidases, peptidases, and nucleosidases.
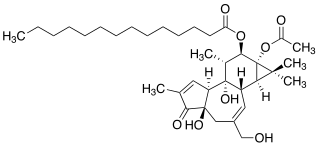
12-O-Tetradecanoylphorbol-13-acetate (TPA), also commonly known as tetradecanoylphorbol acetate, tetradecanoyl phorbol acetate, and phorbol 12-myristate 13-acetate (PMA) is a diester of phorbol. It is a potent tumor promoter often employed in biomedical research to activate the signal transduction enzyme protein kinase C (PKC). The effects of TPA on PKC result from its similarity to one of the natural activators of classic PKC isoforms, diacylglycerol. TPA is a small molecule drug.

URB597 (KDS-4103) is a relatively selective and irreversible inhibitor of the enzyme fatty acid amide hydrolase (FAAH). FAAH is the primary degradatory enzyme for the endocannabinoid anandamide and, as such, inhibition of FAAH leads to an accumulation of anandamide in the CNS and periphery where it activates cannabinoid receptors. URB597 has been found to elevate anandamide levels and have activity against neuropathic pain in a mouse model.
An esterase is a hydrolase enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis.

Phorbol esters are a class of chemical compounds found in a variety of plants, particularly in the families Euphorbiaceae and Thymelaeaceae. Chemically, they are ester derivatives of the tetracyclic diterpenoid phorbol.
In enzymology, a beta-diketone hydrolase (EC 3.7.1.7) is an enzyme that catalyzes the chemical reaction

The enzyme 3-hydroxyisobutyryl-CoA hydrolase (EC 3.1.2.4) catalyzes the reaction
The enzyme acetoacetyl-CoA hydrolase (EC 3.1.2.11) catalyzes the reaction
The enzyme [acyl-carrier-protein] phosphodiesterase (EC 3.1.4.14) catalyzes the reaction
The enzyme CMP-N-acylneuraminate phosphodiesterase (EC 3.1.4.40) catalyzes the reaction
The enzyme glycerophosphodiester phosphodiesterase ({EC 3.1.4.46) catalyzes the reaction
The enzyme hydroxybutyrate-dimer hydrolase (EC 3.1.1.22) catalyzes the reaction
The enzyme sterol esterase (EC 3.1.1.13) catalyzes the reaction
The enzyme wax-ester hydrolase (EC 3.1.1.50) catalyzes the reaction
In enzymology, a gamma-glutamyl-gamma-aminobutyrate hydrolase (EC 3.5.1.94) is an enzyme that catalyzes the chemical reaction

Phorbol 12,13-dibutyrate (PDBu) is a phorbol ester which is one of the constituents of croton oil. As an activator of protein kinase C, it is a weak tumor promoter compared to 12-O-tetradecanoylphorbol-13-acetate.
Asymmetric ester hydrolysis with pig liver esterase is the enantioselective conversion of an ester to a carboxylic acid through the action of the enzyme pig liver esterase. Asymmetric ester hydrolysis involves the selective reaction of one of a pair of either enantiotopic or enantiomorphic ester groups.

Testosterone acetate butyrate, or testosterone 3β-acetate 17β-butanoate, also known as 4-androstenediol acetate butyrate, as well as androst-4-ene-3β,17β-diol 3β-acetate 17β-butanoate, is a synthetic anabolic-androgenic steroid and an androgen ester which was never marketed. It is the 3β-acetate, 17β-butyrate (butanoate) diester of testosterone (androst-4-en-17β-ol-3-one), or, more accurately, of 4-androstenediol (androst-4-ene-3β,17β-diol).
Karen L. Leach is an American biochemist with extensive drug discovery experience in large pharmaceutical research laboratories. Her expertise in molecular pharmacology, signal transduction and protein kinases, has been used to establish mechanisms of toxicity for therapeutics such as the novel antibiotic linezolid (Zyvox).





