Related Research Articles
Polyadenylation is the addition of a poly(A) tail to an RNA transcript, typically a messenger RNA (mRNA). The poly(A) tail consists of multiple adenosine monophosphates; in other words, it is a stretch of RNA that has only adenine bases. In eukaryotes, polyadenylation is part of the process that produces mature mRNA for translation. In many bacteria, the poly(A) tail promotes degradation of the mRNA. It, therefore, forms part of the larger process of gene expression.
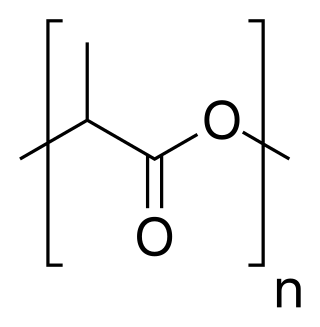
Polylactic acid, also known as poly(lactic acid) or polylactide (PLA), is a thermoplastic polyester with backbone formula (C
3H
4O
2)
n or [–C(CH
3)HC(=O)O–]
n, formally obtained by condensation of lactic acid C(CH
3)(OH)HCOOH with loss of water. It can also be prepared by ring-opening polymerization of lactide [–C(CH
3)HC(=O)O–]
2, the cyclic dimer of the basic repeating unit.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.

β-Hydroxybutyric acid, also known as 3-hydroxybutyric acid or BHB, is an organic compound and a beta hydroxy acid with the chemical formula CH3CH(OH)CH2CO2H; its conjugate base is β-hydroxybutyrate, also known as 3-hydroxybutyrate. β-Hydroxybutyric acid is a chiral compound with two enantiomers: D-β-hydroxybutyric acid and L-β-hydroxybutyric acid. Its oxidized and polymeric derivatives occur widely in nature. In humans, D-β-hydroxybutyric acid is one of two primary endogenous agonists of hydroxycarboxylic acid receptor 2 (HCA2), a Gi/o-coupled G protein-coupled receptor (GPCR).

Monoacylglycerol lipase is an enzyme that, in humans, is encoded by the MGLL gene. MAGL is a 33-kDa, membrane-associated member of the serine hydrolase superfamily and contains the classical GXSXG consensus sequence common to most serine hydrolases. The catalytic triad has been identified as Ser122, His269, and Asp239.

Glycoside hydrolases catalyze the hydrolysis of glycosidic bonds in complex sugars. They are extremely common enzymes with roles in nature including degradation of biomass such as cellulose (cellulase), hemicellulose, and starch (amylase), in anti-bacterial defense strategies, in pathogenesis mechanisms and in normal cellular function. Together with glycosyltransferases, glycosidases form the major catalytic machinery for the synthesis and breakage of glycosidic bonds.
Paucimonas lemoignei, formerly [Pseudomonas lemoignei], is a Gram-negative soil bacterium. It is aerobic, motile, and rod-shaped.
In enzymology, a limonene-1,2-epoxide hydrolase (EC 3.3.2.8) is an enzyme that catalyzes the chemical reaction

The enzyme cutinase is a member of the hydrolase family. It catalyzes the following reaction:
Poly(3-hydroxybutyrate) depolymerase (EC 3.1.1.75, PHB depolymerase, systematic name poly[(R)-3-hydroxybutanoate] hydrolase) is an enzyme used in the degradation processes of a natural polyester poly(3-hydroxyburate). This enzyme has growing commercialization interests due to it implications in biodegradable plastic decomposition.

Endo-polygalacturonase (EC 3.2.1.15, pectin depolymerase, pectolase, pectin hydrolase, and poly-α-1,4-galacturonide glycanohydrolase; systematic name (1→4)-α-D-galacturonan glycanohydrolase (endo-cleaving)) is an enzyme that hydrolyzes the α-1,4 glycosidic bonds between galacturonic acid residues:

Phloretic acid is an organic compound with the formula HOC6H4(CH2)2CO2H. It is a white solid. The compound contains both phenol and carboxylic acid functional groups. It is sometimes called Desaminotyrosine (DAT) because it is identical to the common alpha amino acid tyrosine except for the absence of the amino functional group on the alpha carbon.
Poly( -hydroxyalkanoic acid) depolymerase may refer to:
Exo-poly-α-galacturonosidase is an enzyme with systematic name poly[(1→4)-α-D-galactosiduronate] digalacturonohydrolase. It catalyses the hydrolysis of pectic acid from the non-reducing end, releasing digalacturonate.
Polymannuronate hydrolase is an enzyme with systematic name poly(mannuronide) mannuronohydrolase. It catalyses endohydrolysis of the D-mannuronide linkages of polymannuronate.

Ghk.
Streptomyces exfoliatus is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces exfoliatus has the ability to degrade poly(3-hydroxyalkanoate). This species produces exfoliatin and exfoliamycin.
Ideonella sakaiensis is a bacterium from the genus Ideonella and family Comamonadaceae capable of breaking down and consuming the plastic polyethylene terephthalate (PET) using it as both a carbon and energy source. The bacterium was originally isolated from a sediment sample taken outside of a plastic bottle recycling facility in Sakai City, Japan.
PETases are an esterase class of enzymes that catalyze the breakdown (via hydrolysis) of polyethylene terephthalate (PET) plastic to monomeric mono-2-hydroxyethyl terephthalate (MHET). The idealized chemical reaction is:
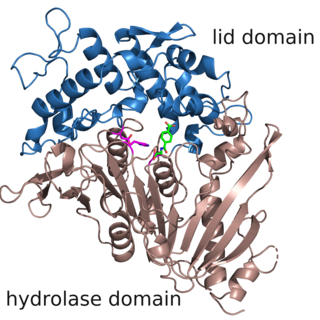
The enzyme MHETase is a hydrolase, which was discovered in 2016. It cleaves 2-hydroxyethyl terephthalic acid, the PET degradation product by PETase, to ethylene glycol and terephthalic acid. This pair of enzymes, PETase and MHETase, enable the bacterium Ideonella sakaiensis to live on the plastic PET as sole carbon source.
References
- Jendrossek D (2001). "Microbial degradation of polyesters". Adv. Biochem. Eng. Biotechnol. 71: 293–325. doi:10.1007/3-540-40021-4_10. PMID 11217416.
- Prieto MA, Garcia JL, Martinez M, Luengo JM (1999). "Novel biodegradable aromatic plastics from a bacterial source Genetic and biochemical studies on a route of the phenylacetyl-CoA catabolon". J. Biol. Chem. 274 (41): 29228–41. doi: 10.1074/jbc.274.41.29228 . PMID 10506180.
- Schirmer A, Jendrossek D, Schlegel HG (1993). "Degradation of poly(3-hydroxyoctanoic acid) [P(3HO)] by bacteria: purification and properties of a P(3HO) depolymerase from Pseudomonas fluorescens GK13". Appl. Environ. Microbiol. 59 (4): 1220–7. PMC 202264 . PMID 8476295.