
Vitamin A is a fat-soluble vitamin and an essential nutrient for animals. The term "vitamin A" encompasses a group of chemically related organic compounds that includes retinol, retinal, retinoic acid, and several provitamin (precursor) carotenoids, most notably beta-carotene. Vitamin A has multiple functions: it is essential for embryo development and growth, for maintenance of the immune system, and for vision, where it combines with the protein opsin to form rhodopsin – the light-absorbing molecule necessary for both low-light and color vision.
In biochemistry, hydrolases constitute a class of enzymes that commonly function as biochemical catalysts that use water to break a chemical bond:

The retinoids are a class of chemical compounds that are vitamers of vitamin A or are chemically related to it. Retinoids have found use in medicine where they regulate epithelial cell growth.

Retinyl palmitate, or vitamin A palmitate, is the ester of retinol (vitamin A) and palmitic acid, with formula C36H60O2. It is the most abundant form of vitamin A storage in animals.

Vitamin A deficiency (VAD) or hypovitaminosis A is a lack of vitamin A in blood and tissues. It is common in poorer countries, especially among children and women of reproductive age, but is rarely seen in more developed countries. Nyctalopia is one of the first signs of VAD, as the vitamin has a major role in phototransduction; but it is also the first symptom that is reversed when vitamin A is consumed again. Xerophthalmia, keratomalacia, and complete blindness can follow if the deficiency is more severe.
The visual cycle is a process in the retina that replenishes the molecule retinal for its use in vision. Retinal is the chromophore of most visual opsins, meaning it captures the photons to begin the phototransduction cascade. When the photon is absorbed, the 11-cis retinal photoisomerizes into all-trans retinal as it is ejected from the opsin protein. Each molecule of retinal must travel from the photoreceptor cell to the RPE and back in order to be refreshed and combined with another opsin. This closed enzymatic pathway of 11-cis retinal is sometimes called Wald's visual cycle after George Wald (1906–1997), who received the Nobel Prize in 1967 for his work towards its discovery.
The enzyme 11-cis-retinyl-palmitate hydrolase (EC 3.1.1.63) catalyzes the reaction

The enzyme 3-hydroxyisobutyryl-CoA hydrolase (EC 3.1.2.4) catalyzes the reaction
In biochemistry, an acetylesterase is a class of enzyme which catalyzes the hydrolysis of acetic esters into an alcohol and acetic acid:
The enzyme acylcarnitine hydrolase (EC 3.1.1.28) catalyzes the reaction
The enzyme retinoid isomerohydrolase (EC 3.1.1.64, all-trans-retinyl-palmitate hydrolase) catalyzes the reaction

Palmitoyl protein hydrolase/thioesterases is an enzyme (EC 3.1.2.22) that removes thioester-linked fatty acyl groups such as palmitate from modified cysteine residues in proteins or peptides during lysosomal degradation. It catalyzes the reaction
The enzyme wax-ester hydrolase (EC 3.1.1.50) catalyzes the reaction
In enzymology, a phosphatidylcholine---retinol O-acyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a polysialic-acid O-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a retinol O-fatty-acyltransferase is an enzyme that catalyzes the chemical reaction

Retinal pigment epithelium-specific 65 kDa protein, also known as retinoid isomerohydrolase, is an enzyme of the vertebrate visual cycle that is encoded in humans by the RPE65 gene. RPE65 is expressed in the retinal pigment epithelium and is responsible for the conversion of all-trans-retinyl esters to 11-cis-retinol during phototransduction. 11-cis-retinol is then used in visual pigment regeneration in photoreceptor cells. RPE65 belongs to the carotenoid oxygenase family of enzymes.
Retinyl acetate is a natural form of vitamin A which is the acetate ester of retinol. It has potential antineoplastic and chemopreventive activities.
The enzyme all-trans-retinyl ester 13-cis isomerohydrolase (EC 3.1.1.90; systematic name all-trans-retinyl ester acylhydrolase, 13-cis retinol forming catalyses the reaction
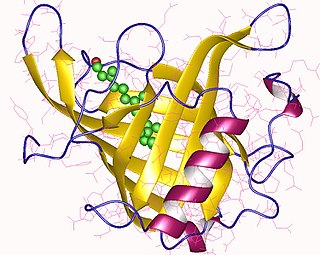
Retinol-binding proteins (RBP) are a family of proteins with diverse functions. They are carrier proteins that bind retinol. Assessment of retinol-binding protein is used to determine visceral protein mass in health-related nutritional studies.








