
In cell biology, protein kinase A (PKA) is a family of serine-threonine kinase whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase. PKA has several functions in the cell, including regulation of glycogen, sugar, and lipid metabolism. It should not be confused with 5'-AMP-activated protein kinase.
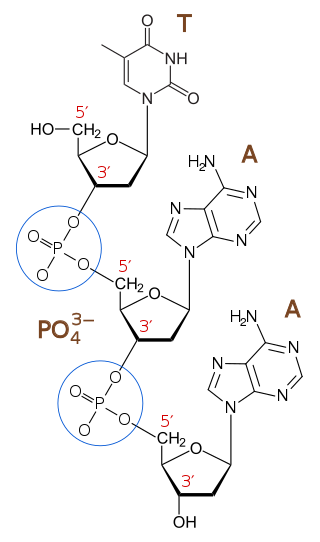
In chemistry, a phosphodiester bond occurs when exactly two of the hydroxyl groups in phosphoric acid react with hydroxyl groups on other molecules to form two ester bonds. The "bond" involves this linkage C−O−PO−2O−C. Discussion of phosphodiesters is dominated by their prevalence in DNA and RNA, but phosphodiesters occur in other biomolecules, e.g. acyl carrier proteins.
Hydrolase is a class of enzymes that commonly perform as biochemical catalysts that use water to break a chemical bond, which typically results in dividing a larger molecule into smaller molecules. Some common examples of hydrolase enzymes are esterases including lipases, phosphatases, glycosidases, peptidases, and nucleosidases.
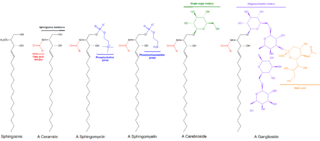
Sphingolipids are a class of lipids containing a backbone of sphingoid bases, which are a set of aliphatic amino alcohols that includes sphingosine. They were discovered in brain extracts in the 1870s and were named after the mythological sphinx because of their enigmatic nature. These compounds play important roles in signal transduction and cell recognition. Sphingolipidoses, or disorders of sphingolipid metabolism, have particular impact on neural tissue. A sphingolipid with a terminal hydroxyl group is a ceramide. Other common groups bonded to the terminal oxygen atom include phosphocholine, yielding a sphingomyelin, and various sugar monomers or dimers, yielding cerebrosides and globosides, respectively. Cerebrosides and globosides are collectively known as glycosphingolipids.

3′,5′-cyclic-nucleotide phosphodiesterases (EC 3.1.4.17) are a family of phosphodiesterases. Generally, these enzymes hydrolyze a nucleoside 3′,5′-cyclic phosphate to a nucleoside 5′-phosphate:
In enzymology, a phosphoethanolamine N-methyltransferase is an enzyme that catalyzes the chemical reaction
The enzyme sphinganine-1-phosphate aldolase catalyzes the chemical reaction
The enzyme ethanolamine-phosphate phospho-lyase (EC 4.2.3.2) catalyzes the chemical reaction
The enzyme 2′,3′-cyclic-nucleotide 2'-phosphodiesterase (EC 3.1.4.16) catalyzes the reaction
The enzyme 3′,5′-cyclic-GMP phosphodiesterase (EC 3.1.4.35) catalyzes the reaction
The enzyme alkylglycerophosphoethanolamine phosphodiesterase (EC 3.1.4.39) catalyzes the reaction
In the field of enzymology, a glycerophospholipid arachidonoyl-transferase (CoA-independent) is an enzyme that catalyzes the chemical reaction:
Choline kinase is an enzyme which catalyzes the first reaction in the choline pathway for phosphatidylcholine (PC) biosynthesis. This reaction involves the transfer of a phosphate group from adenosine triphosphate (ATP) to choline in order to form phosphocholine.
In enzymology, an ethanolamine kinase is an enzyme that catalyzes the chemical reaction
In enzymology, an ethanolamine-phosphate cytidylyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a serine-phosphoethanolamine synthase is an enzyme that catalyzes the chemical reaction
The enzyme protein serine/threonine phosphatase is a form of phosphoprotein phosphatase that acts upon phosphorylated serine/threonine residues:
Glypiation is the addition by covalent bonding of a glycosylphosphatidylinositol (GPI) anchor and is a common post-translational modification that localizes proteins to cell membranes. This special kind of glycosylation is widely detected on surface glycoproteins in eukaryotes and some Archaea.
Phosphoethanolamine/phosphocholine phosphatase (EC 3.1.3.75, PHOSPHO1, 3X11A; systematic name phosphoethanolamine phosphohydrolase) is an enzyme highly expressed in mineralizing cells. This enzyme is implicated in bone and cartilage formation and catalyses the following chemical reactions:
In enzymology, a ceramide phosphoethanolamine synthase is an enzyme that catalyzes the chemical reaction





