
Atropine is a tropane alkaloid and anticholinergic medication used to treat certain types of nerve agent and pesticide poisonings as well as some types of slow heart rate, and to decrease saliva production during surgery. It is typically given intravenously or by injection into a muscle. Eye drops are also available which are used to treat uveitis and early amblyopia. The intravenous solution usually begins working within a minute and lasts half an hour to an hour. Large doses may be required to treat some poisonings.
Hydrolase is a class of enzymes that commonly perform as biochemical catalysts that use water to break a chemical bond, which typically results in dividing a larger molecule into smaller molecules. Some common examples of hydrolase enzymes are esterases including lipases, phosphatases, glycosidases, peptidases, and nucleosidases.

Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commodity during World War I. Tropinone and the alkaloids cocaine and atropine all share the same tropane core structure. Its corresponding conjugate acid at pH 7.3 major species is known as tropiniumone.
Tropane is a nitrogenous bicyclic organic compound. It is mainly known for the other alkaloids derived from it, which include atropine and cocaine, among others. Tropane alkaloids occur in plants of the families Erythroxylaceae and Solanaceae.
Tropine is a derivative of tropane containing a hydroxyl group at the third carbon. It is also called 3-tropanol. It is a poisonous white hygroscopic crystalline powder. It is a heterocyclic alcohol and an amine.
In enzymology, a tropinone reductase I (EC 1.1.1.206) is an enzyme that catalyzes the chemical reaction
In enzymology, a tropinone reductase II (EC 1.1.1.236) is an enzyme that catalyzes the chemical reaction
The enzyme 1-alkyl-2-acetylglycerophosphocholine esterase (EC 3.1.1.47) catalyzes the reaction
The enzyme acetoxybutynylbithiophene deacetylase (EC 3.1.1.54) catalyzes the reaction
The enzyme acetyl-CoA hydrolase catalyzes the reaction
The enzyme carboxylesterase (or carboxylic-ester hydrolase, EC 3.1.1.1; systematic name carboxylic-ester hydrolase) catalyzes reactions of the following form:
The enzyme lysophospholipase (EC 3.1.1.5) catalyzes the reaction

The enzyme polyneuridine-aldehyde esterase (EC 3.1.1.78) catalyzes the following reaction:
The enzyme S-formylglutathione hydrolase (EC 3.1.2.12) catalyzes the reaction
The enzyme sterol esterase (EC 3.1.1.13) catalyzes the reaction
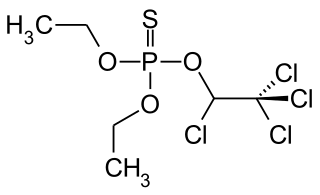
Chlorethoxyfos is an organophosphate acetylcholinesterase inhibitor used as an insecticide. It is registered for the control of corn rootworms, wireworms, cutworms, seed corn maggot, white grubs and symphylans on corn. The insecticide is sold under the trade name Fortress by E.I. du Pont de Nemours & Company.

Disulfoton is an organophosphate acetylcholinesterase inhibitor used as an insecticide. It is manufactured under the name Di-Syston by Bayer CropScience. Disulfoton in its pure form is a colorless oil but the technical product used in vegetable fields is dark and yellowish with a sulfur odor. Disulfoton is processed as a liquid into carrier granules, these granules are mixed with fertilizer and clay to be made into a spike, designed to be driven into the ground. The pesticide is absorbed over time by the roots and translocated to all parts of the plant. The pesticide acts as a cholinesterase inhibitor and gives long lasting control.
Asymmetric ester hydrolysis with pig liver esterase is the enantioselective conversion of an ester to a carboxylic acid through the action of the enzyme pig liver esterase. Asymmetric ester hydrolysis involves the selective reaction of one of a pair of either enantiotopic or enantiomorphic ester groups.
(S)-hydroxynitrile lyase (EC 4.1.2.47, (S)-cyanohydrin producing hydroxynitrile lyase, (S)-oxynitrilase, (S)-HbHNL, (S)-MeHNL, hydroxynitrile lyase, oxynitrilase, HbHNL, MeHNL, (S)-selective hydroxynitrile lyase, (S)-cyanohydrin carbonyl-lyase (cyanide forming), hydroxynitrilase) is an enzyme with systematic name (S)-cyanohydrin lyase (cyanide forming). This enzyme catalyses the interconversion between cyanohydrins and the carbonyl compounds derived from the cyanohydrin with free cyanide, as in the following two chemical reactions:

An O-acylpseudotropine is any derivative of pseudotropine in which the alcohol group is substituted with an acyl group.






