
A catalytic triad is a set of three coordinated amino acid residues that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes. An acid-base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to release the product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine, but occasionally threonine or even selenocysteine. The 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence.

Sodium monofluorophosphate, commonly abbreviated SMFP, is an inorganic compound with the chemical formula Na2PO3F. Typical for a salt, SMFP is odourless, colourless, and water-soluble. This salt is an ingredient in some toothpastes.

Silver oxide is the chemical compound with the formula Ag2O. It is a fine black or dark brown powder that is used to prepare other silver compounds.
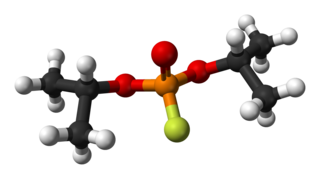
Diisopropyl fluorophosphate (DFP) or Isoflurophate is an oily, colorless liquid with the chemical formula C6H14FO3P. It is used in medicine and as an organophosphorus insecticide. It is stable, but undergoes hydrolysis when subjected to moisture.

Methoxy arachidonyl fluorophosphonate, commonly referred as MAFP, is an irreversible active site-directed enzyme inhibitor that inhibits nearly all serine hydrolases and serine proteases. It inhibits phospholipase A2 and fatty acid amide hydrolase with special potency, displaying IC50 values in the low-nanomolar range. In addition, it binds to the CB1 receptor in rat brain membrane preparations (IC50 = 20 nM), but does not appear to agonize or antagonize the receptor, though some related derivatives do show cannabinoid-like properties.

Leukotriene-A4 hydrolase is an enzyme that catalyzes the reaction which converts Leukotriene A4 to Leukotriene B4. It is a bifunctional zinc enzyme with different amino acids attached to it to aid in the catalysis of the reaction. It also acts as an aminopeptidase. Leukotriene-A4 hydrolase is a cytosolic protein and is found in almost all mammalian cells, tissues and organelles that have been examined.
The enzyme aspartate ammonia-lyase (EC 4.3.1.1) catalyzes the chemical reaction
The enzyme orsellinate decarboxylase (EC 4.1.1.58) catalyzes the chemical reaction
In enzymology, an endopolyphosphatase (EC 3.6.1.10) is an enzyme that catalyzes the chemical reaction
In biochemistry, an acetylesterase is a class of enzyme which catalyzes the hydrolysis of acetic esters into an alcohol and acetic acid:
The enzyme tannase (EC 3.1.1.20) catalyzes the following reaction:
In enzymology, an ADP deaminase (EC 3.5.4.7) is an enzyme that catalyzes the chemical reaction
Decylcitrate synthase (EC 2.3.3.2) is an enzyme that catalyzes the chemical reaction in enzymology.

Carbonic anhydrase 4 is an enzyme that in humans is encoded by the CA4 gene.

Iodine pentoxide is the chemical compound with the formula I2O5. This iodine oxide is the anhydride of iodic acid, and one of the few iodine oxides that is stable. It is produced by dehydrating iodic acid at 200 °C in a stream of dry air:

Neutral cholesterol ester hydrolase 1 (NCEH) also known as arylacetamide deacetylase-like 1 (AADACL1) or KIAA1363 is an enzyme that in humans is encoded by the NCEH1 gene.
Organophosphorus acid anhydrolase (OPAA) is an enzyme that been shown to be particularly effective in detoxifying organophosphorus-containing compounds, such as deadly nerve gas used in chemical warfare. The enzyme is found in a diverse range of organisms, including protozoa, squid and clams, mammals, and soil bacteria. A highly active form of the enzyme is typically isolated from the marine bacteria Alteromonas undina for laboratory study. This form is both halophilic and thermophilic, making it particularly useful for detoxification applications. A slightly less active variant of OPAA has also been isolated in mung beans and slime mold duckweed.
Gösta Pettersson is an emeritus professor in biochemistry at Lund University, Sweden. He was born in 1937 in Varberg, Sweden. He gained his Ph.D. at Lund University in 1966 on the basis of a thesis on toluquinones, and his early research was mainly concerned with fumigatin and other products of fungal metabolism.

Methylfluorophosphonylcholine (MFPCh) is an extremely toxic chemical compound related to the G-series nerve agents. It is an extremely potent acetylcholinesterase inhibitor which is around 100 times more potent than sarin at inhibiting acetylcholinesterase in vitro, and around 10 times more potent in vivo, depending on route of administration and animal species tested. MFPCh is resistant to oxime reactivators, meaning the acetylcholinesterase inhibited by MFPCh can't be reactivated by cholinesterase reactivators. MFPCh also acts directly on the acetylcholine receptors. MFPCh is a relatively unstable compound and degrades rapidly in storage, so despite its enhanced toxicity it was not deemed suitable to be weaponised for military use.

Nitratoauric acid, hydrogen tetranitratoaurate, or simply called gold(III) nitrate is a crystalline gold compound that forms the trihydrate, HAu(NO3)4·3H2O or more correctly H5O2Au(NO3)4·H2O. This compound is an intermediate in the process of extracting gold. In older literature it is also known as aurinitric acid.













