Related Research Articles
Enoyl-CoA-(∆) isomerase (EC 5.3.3.8, also known as dodecenoyl-CoA- isomerase, 3,2-trans-enoyl-CoA isomerase, ∆3 ,∆2 -enoyl-CoA isomerase, or acetylene-allene isomerase, is an enzyme that catalyzes the conversion of cis- or trans-double bonds of coenzyme A bound fatty acids at gamma-carbon to trans double bonds at beta-carbon as below:

Trypanothione is an unusual form of glutathione containing two molecules of glutathione joined by a spermidine (polyamine) linker. It is found in parasitic protozoa such as leishmania and trypanosomes. These protozoal parasites are the cause of leishmaniasis, sleeping sickness and Chagas' disease. Trypanothione was discovered by Alan Fairlamb. Its structure was proven by chemical synthesis. It is present mainly in the Kinetoplastida but can be found in other parasitic protozoa such as Entamoeba histolytica. Since this thiol is absent from humans and is essential for the survival of the parasites, the enzymes that make and use this molecule are targets for the development of new drugs to treat these diseases.
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds. The study of drug metabolism is called pharmacokinetics.

Glutathione disulfide (GSSG) is a disulfide derived from two glutathione molecules.

A catalytic triad is a set of three coordinated amino acids that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes. An acid-base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to release the product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine or even selenocysteine. The 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence.
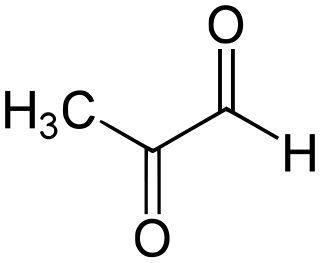
Methylglyoxal (MGO) is the organic compound with the formula CH3C(O)CHO. It is a reduced derivative of pyruvic acid. It is a reactive compound that is implicated in the biology of diabetes. Methylglyoxal is produced industrially by degradation of carbohydrates using overexpressed methylglyoxal synthase.

Glutathione reductase (GR) also known as glutathione-disulfide reductase (GSR) is an enzyme that in humans is encoded by the GSR gene. Glutathione reductase catalyzes the reduction of glutathione disulfide (GSSG) to the sulfhydryl form glutathione (GSH), which is a critical molecule in resisting oxidative stress and maintaining the reducing environment of the cell. Glutathione reductase functions as dimeric disulfide oxidoreductase and utilizes an FAD prosthetic group and NADPH to reduce one molar equivalent of GSSG to two molar equivalents of GSH:
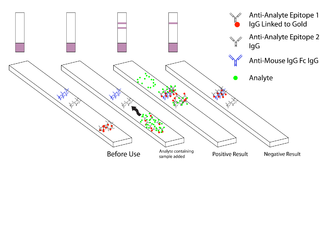
Malaria antigen detection tests are a group of commercially available rapid diagnostic tests of the rapid antigen test type that allow quick diagnosis of malaria by people who are not otherwise skilled in traditional laboratory techniques for diagnosing malaria or in situations where such equipment is not available. There are currently over 20 such tests commercially available. The first malaria antigen suitable as target for such a test was a soluble glycolytic enzyme Glutamate dehydrogenase. None of the rapid tests are currently as sensitive as a thick blood film, nor as cheap. A major drawback in the use of all current dipstick methods is that the result is essentially qualitative. In many endemic areas of tropical Africa, however, the quantitative assessment of parasitaemia is important, as a large percentage of the population will test positive in any qualitative assay.

Glutathione S-transferase A1 is an enzyme that in humans is encoded by the GSTA1 gene.
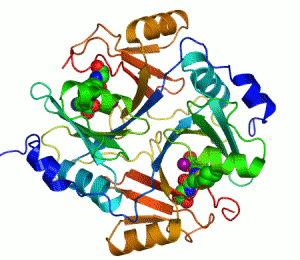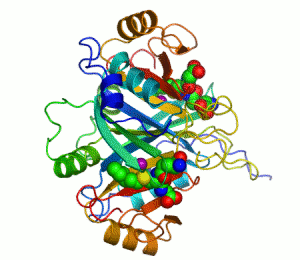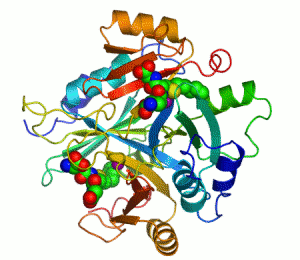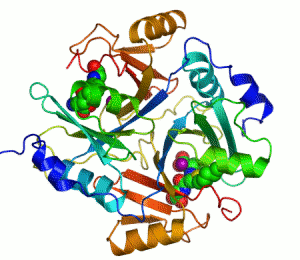
The enzyme lactoylglutathione lyase (EC 4.4.1.5, also known as glyoxalase I) catalyzes the isomerization of hemithioacetal adducts, which are formed in a spontaneous reaction between a glutathionyl group and aldehydes such as methylglyoxal.
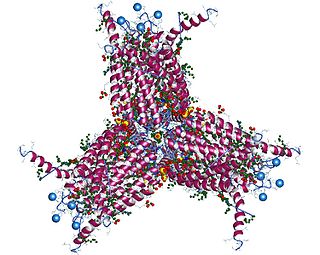
The enzyme leukotriene-C4 synthase (EC 4.4.1.20) catalyzes the reaction
The glyoxalase system is a set of enzymes that carry out the detoxification of methylglyoxal and the other reactive aldehydes that are produced as a normal part of metabolism. This system has been studied in both bacteria and eukaryotes. This detoxification is accomplished by the sequential action of two thiol-dependent enzymes; firstly glyoxalase І, which catalyzes the isomerization of the spontaneously formed hemithioacetal adduct between glutathione and 2-oxoaldehydes into S-2-hydroxyacylglutathione. Secondly, glyoxalase ІІ hydrolyses these thiolesters and in the case of methylglyoxal catabolism, produces D-lactate and GSH from S-D-lactoyl-glutathione.
The enzyme carboxylesterase (or carboxylic-ester hydrolase, EC 3.1.1.1; systematic name carboxylic-ester hydrolase) catalyzes reactions of the following form:
The enzyme hydroxymethylglutaryl-CoA hydrolase (EC 3.1.2.5) catalyzes the reaction
Liver carboxylesterase 1 also known as carboxylesterase 1 is an enzyme that in humans is encoded by the CES1 gene. The protein is also historically known as serine esterase 1 (SES1), monocyte esterase and cholesterol ester hydrolase (CEH). Three transcript variants encoding three different isoforms have been found for this gene. The various protein products from isoform a, b and c range in size from 568, 567 and 566 amino acids long, respectively.

Glutathione S-transferase A2 is an enzyme that in humans is encoded by the GSTA2 gene.
Hydroxyacylglutathione hydrolase, mitochondrial is an enzyme that in humans is encoded by the HAGH gene.

Glutathione S-transferase A4, also known as GSTA4, is an enzyme which in humans is encoded by the GSTA4 gene.

Epoxide hydrolase 1 is an enzyme encoded by the EPHX1 gene in humans.

3-Deoxyglucosone (3DG) is a sugar that is notable because it is a marker for diabetes. 3DG reacts with protein to form advanced glycation end-products (AGEs), which contribute to diseases such as the vascular complications of diabetes, atherosclerosis, hypertension, Alzheimer's disease, inflammation, and aging.
References
- ↑ Vander Jagt DL (1993). "Glyoxalase II: molecular characteristics, kinetics and mechanism". Biochem. Soc. Trans. 21 (2): 522–7. doi:10.1042/bst0210522. PMID 8359524.
- Ball JC, Vander Jagt DL (1979). "Purification of S-2-hydroxyacylglutathione hydrolase (glyoxalase II) from rat erythrocytes". Anal. Biochem. 98 (2): 472–7. doi:10.1016/0003-2697(79)90169-6. PMID 496013.