
Epoxide hydrolases (EHs), also known as epoxide hydratases, are enzymes that metabolize compounds that contain an epoxide residue; they convert this residue to two hydroxyl residues through an epoxide hydrolysis reaction to form diol products. Several enzymes possess EH activity. Microsomal epoxide hydrolase, soluble epoxide hydrolase, and the more recently discovered but not as yet well defined functionally, epoxide hydrolase 3 (EH3) and epoxide hydrolase 4 (EH4) are structurally closely related isozymes. Other enzymes with epoxide hydrolase activity include leukotriene A4 hydrolase, Cholesterol-5,6-oxide hydrolase, MEST (gene) (Peg1/MEST), and Hepoxilin-epoxide hydrolase. The hydrolases are distinguished from each other by their substrate preferences and, directly related to this, their functions.

Lipid signaling, broadly defined, refers to any biological signaling event involving a lipid messenger that binds a protein target, such as a receptor, kinase or phosphatase, which in turn mediate the effects of these lipids on specific cellular responses. Lipid signaling is thought to be qualitatively different from other classical signaling paradigms because lipids can freely diffuse through membranes. One consequence of this is that lipid messengers cannot be stored in vesicles prior to release and so are often biosynthesized "on demand" at their intended site of action. As such, many lipid signaling molecules cannot circulate freely in solution but, rather, exist bound to special carrier proteins in serum.
In enzymology, a vitamin-K-epoxide reductase (warfarin-insensitive) is an enzyme that catalyzes the chemical reaction
In enzymology, a vitamin-K-epoxide reductase (warfarin-sensitive) is an enzyme that catalyzes the chemical reaction
In enzymology, a hepoxilin-epoxide hydrolase is an enzyme that catalyzes the conversion of the epoxyalcohol metabolites arachidonic acid, hepoxilin A3 and hepoxilin B3 to their tri-hydroxyl products, trioxolin A3 and trioxilin B3, respectively. These reactions in general inactivate the two biologically active hepoxilins.

Leukotriene A4 hydrolase, also known as LTA4H is a human gene. The protein encoded by this gene is a bifunctional enzyme which converts leukotriene A4 to leukotriene B4 and acts as an aminopeptidase.

In enzymology, a microsomal epoxide hydrolase (mEH) is an enzyme that catalyzes the hydrolysis reaction between an epoxide and water to form a diol.
UDP-sulfoquinovose synthase (EC 3.13.1.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a dolichyl-phosphate beta-glucosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a dephospho-[reductase kinase] kinase is an enzyme that catalyzes the chemical reaction

Mannose-6-phosphate receptor binding protein 1 (M6PRBP1) is a protein which in humans is encoded by the M6PRBP1 gene. Its gene product, as well as the gene itself, is commonly known as TIP47.

RNA polymerase II subunit A C-terminal domain phosphatase is an enzyme that in humans is encoded by the CTDP1 gene.

Lipid phosphate phosphohydrolase 1 also known as phosphatidic acid phosphatase 2a is an enzyme that in humans is encoded by the PPAP2A gene.
Myotubularin domain represents a region within eukaryotic myotubularin-related proteins that is sometimes found with the GRAM domain InterPro: IPR004182. Myotubularin is a dual-specific lipid phosphatase that dephosphorylates phosphatidylinositol 3-phosphate and phosphatidylinositol (3,5)-bi-phosphate. Mutations in gene encoding myotubularin-related proteins have been associated with disease.

Cytochrome P450 4F8 is a protein that in humans is encoded by the CYP4F8 gene.

Cytochrome P450 4F12 is a protein that in humans is encoded by the CYP4F12 gene.

Sphingosine-1-phosphate phosphatase 1 is an enzyme that in humans is encoded by the SGPP1 gene.

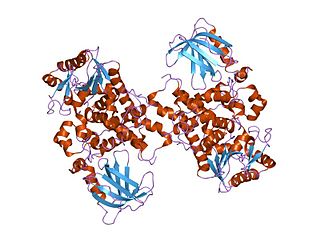
Soluble epoxide hydrolase (sEH) is a bifunctional enzyme that in humans is encoded by the EPHX2 gene. sEH is a member of the epoxide hydrolase family. This enzyme, found in both the cytosol and peroxisomes, binds to specific epoxides and converts them to the corresponding diols. A different region of this protein also has lipid-phosphate phosphatase activity. Mutations in the EPHX2 gene have been associated with familial hypercholesterolemia.
Cholesterol-5,6-oxide hydrolase (EC 3.3.2.11, cholesterol-epoxide hydrolase, ChEH) is an enzyme with systematic name 5,6alpha-epoxy-5alpha-cholestan-3beta-ol hydrolase. This enzyme catalyses the following chemical reaction

Epoxide hydrolase 3, encoded by the EPHX3 gene, is the third defined isozyme in a set of epoxide hydrolase isozymes, i.e. the epoxide hydrolases. This set includes the Microsomal epoxide hydrolase ; the epoxide hydrolase 2 ; and the far less well defined enzymatically, epoxide hydrolase 4. All four enzyme contain an Alpha/beta hydrolase fold suggesting that they have Hydrolysis activity. EH1, EH2, and EH3 have been shown to have such activity in that they add water to epoxides of unsaturated fatty acids to form vicinal cis products; the activity of EH4 has not been reported. The former three EH's differ in subcellular location, tissue expression patterns, substrate preferences, and thereby functions. These functions include limiting the biologically actions of certain fatty acid epoxides, increasing the toxicity of other fatty acid epoxides, and contributing to the metabolism of drugs and other xenobiotics.










