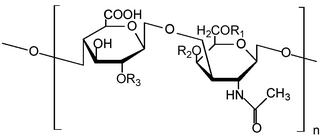
Glycosaminoglycans (GAGs) or mucopolysaccharides are long, linear polysaccharides consisting of repeating disaccharide units. The repeating two-sugar unit consists of a uronic sugar and an amino sugar, except in the case of the sulfated glycosaminoglycan keratan, where, in place of the uronic sugar there is a galactose unit. GAGs are found in vertebrates, invertebrates and bacteria. Because GAGs are highly polar molecules and attract water; the body uses them as lubricants or shock absorbers.

Sulfur assimilation is the process by which living organisms incorporate sulfur into their biological molecules. In plants, sulfate is absorbed by the roots and then be transported to the chloroplasts by the transipration stream where the sulfur are reduced to sulfide with the help of a series of enzymatic reactions. Furthermore, the reduced sulfur is incorporated into cysteine, an amino acid that is a precursor to many other sulfur-containing compounds. In animals, sulfur assimilation occurs primarily through the diet, as animals cannot produce sulfur-containing compounds directly. Sulfur is incorporated into amino acids such as cysteine and methionine, which are used to build proteins and other important molecules. Besides, With the rapid development of economy, the increase emission of sulfur results in environmental issues, such as acid rain and hydrogen sulfilde.

Heparan sulfate (HS) is a linear polysaccharide found in all animal tissues. It occurs as a proteoglycan in which two or three HS chains are attached in close proximity to cell surface or extracellular matrix proteins. In this form, HS binds to a variety of protein ligands, including Wnt, and regulates a wide range of biological activities, including developmental processes, angiogenesis, blood coagulation, abolishing detachment activity by GrB, and tumour metastasis. HS has also been shown to serve as cellular receptor for a number of viruses, including the respiratory syncytial virus. One study suggests that cellular heparan sulfate has a role in SARS-CoV-2 Infection, particularly when the virus attaches with ACE2.

Steroid sulfatase (STS), or steryl-sulfatase, formerly known as arylsulfatase C, is a sulfatase enzyme involved in the metabolism of steroids. It is encoded by the STS gene.
Sulfatases EC 3.1.6.- are enzymes of the esterase class that catalyze the hydrolysis of sulfate esters. These may be found on a range of substrates, including steroids, carbohydrates and proteins. Sulfate esters may be formed from various alcohols and amines. In the latter case the resultant N-sulfates can also be termed sulfamates.

Arylsulfatase B is an enzyme associated with mucopolysaccharidosis VI.
Arylsulfatase (EC 3.1.6.1, sulfatase, nitrocatechol sulfatase, phenolsulfatase, phenylsulfatase, p-nitrophenyl sulfatase, arylsulfohydrolase, 4-methylumbelliferyl sulfatase, estrogen sulfatase) is a type of sulfatase enzyme with systematic name aryl-sulfate sulfohydrolase. This enzyme catalyses the following chemical reaction
Translocase is a general term for a protein that assists in moving another molecule, usually across a cell membrane. These enzymes catalyze the movement of ions or molecules across membranes or their separation within membranes. The reaction is designated as a transfer from “side 1” to “side 2” because the designations “in” and “out”, which had previously been used, can be ambiguous. Translocases are the most common secretion system in Gram positive bacteria.

N-acetylglucosamine-6-sulfatase (EC 3.1.6.14, glucosamine (N-acetyl)-6-sulfatase, systematic name N-acetyl-D-glucosamine-6-sulfate 6-sulfohydrolase) is an enzyme that in humans is encoded by the GNS gene. It is deficient in Sanfilippo Syndrome type IIId. It catalyses the hydrolysis of the 6-sulfate groups of the N-acetyl-D-glucosamine 6-sulfate units of heparan sulfate and keratan sulfate

Tyrosylprotein sulfotransferase is an enzyme that catalyzes tyrosine sulfation.
An aryl sulfotransferase is an enzyme that transfers a sulfate group from phenolic sulfate esters to a phenolic acceptor substrate.
Estrone sulfotransferase (EST), also known as estrogen sulfotransferase, is an enzyme that catalyzes the transformation of an unconjugated estrogen like estrone into a sulfated estrogen like estrone sulfate. It is a steroid sulfotransferase and belongs to the family of transferases, to be specific, the sulfotransferases, which transfer sulfur-containing groups. This enzyme participates in androgen and estrogen metabolism and sulfur metabolism.
In enzymology, a steroid sulfotransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an adenylylsulfatase is an enzyme that catalyzes the chemical reaction
In enzymology, a phosphoadenylylsulfatase (EC 3.6.2.2) is an enzyme that catalyzes the chemical reaction

Pregnenolone sulfate is an endogenous excitatory neurosteroid that is synthesized from pregnenolone. It is known to have cognitive and memory-enhancing, antidepressant, anxiogenic, and proconvulsant effects.

Quercetin 3-sulfate is a plasma human metabolite of quercetin. It is the sulfate conjugate of quercetin.

Magnesium-protoporphyrin IX monomethyl ester (oxidative) cyclase, is an enzyme with systematic name magnesium-protoporphyrin-IX 13-monomethyl ester, ferredoxin:oxygen oxidoreductase (hydroxylating). In plants this enzyme catalyses the following overall chemical reaction

Chlorophyllide a and Chlorophyllide b are the biosynthetic precursors of chlorophyll a and chlorophyll b respectively. Their propionic acid groups are converted to phytyl esters by the enzyme chlorophyll synthase in the final step of the pathway. Thus the main interest in these chemical compounds has been in the study of chlorophyll biosynthesis in plants, algae and cyanobacteria. Chlorophyllide a is also an intermediate in the biosynthesis of bacteriochlorophylls.
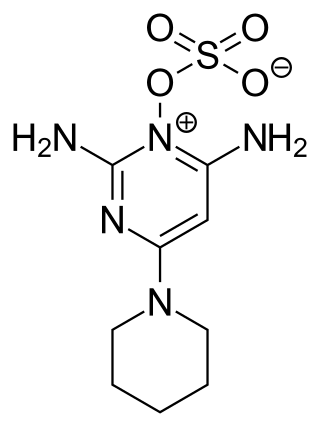
Minoxidil sulfate, also known as minoxidil sulfate ester or minoxidil N-O-sulfate, is an active metabolite of minoxidil and is the active form of this agent. Minoxidil acts as a prodrug of minoxidil sulfate. Minoxidil sulfate is formed from minoxidil via sulfotransferase enzymes, with the predominant enzyme responsible, at least in hair follicles, being SULT1A1. Minoxidil sulfate acts as a potassium channel opener, among other actions, and has vasodilating, hypotensive, and trichogenic or hypertrichotic effects. Its mechanism of action in terms of hair growth is still unknown, although multiple potential mechanisms have been implicated.














