Hydrolysis is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.

In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a P–O–P linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate (Na2H2P2O7) and tetrasodium pyrophosphate (Na4P2O7), among others. Often pyrophosphates are called diphosphates. The parent pyrophosphates are derived from partial or complete neutralization of pyrophosphoric acid. The pyrophosphate bond is also sometimes referred to as a phosphoanhydride bond, a naming convention which emphasizes the loss of water that occurs when two phosphates form a new P–O–P bond, and which mirrors the nomenclature for anhydrides of carboxylic acids. Pyrophosphates are found in ATP and other nucleotide triphosphates, which are important in biochemistry. The term pyrophosphate is also the name of esters formed by the condensation of a phosphorylated biological compound with inorganic phosphate, as for dimethylallyl pyrophosphate. This bond is also referred to as a high-energy phosphate bond.
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually containing oxygen. When combined with the hydrocarbon terpenes, terpenoids comprise about 80,000 compounds. They are the largest class of plant secondary metabolites, representing about 60% of known natural products. Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists.

Sabinene is a natural bicyclic monoterpene with the molecular formula C10H16. It is isolated from the essential oils of a variety of plants including Marjoram, holm oak (Quercus ilex) and Norway spruce (Picea abies). It has a strained ring system with a cyclopentane ring fused to a cyclopropane ring.

Geranyl pyrophosphate (GPP), also known as geranyl diphosphate (GDP), is the pyrophosphate ester of the terpenoid geraniol. Its salts are colorless. It is a precursor to many natural products.
Farnesyl pyrophosphate (FPP), also known as farnesyl diphosphate (FDP), is an intermediate in the biosynthesis of terpenes and terpenoids such as sterols and carotenoids. It is also used in the synthesis of CoQ, as well as dehydrodolichol diphosphate.
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries.
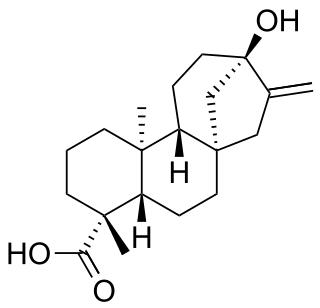
Steviol is a diterpene first isolated from the plant Stevia rebaudiana in 1931. Its chemical structure was not fully elucidated until 1960.
Pyrophosphatases, also known as diphosphatases, are acid anhydride hydrolases that act upon diphosphate bonds.

Isopentenyl pyrophosphate isomerase, also known as Isopentenyl-diphosphate delta isomerase, is an isomerase that catalyzes the conversion of the relatively un-reactive isopentenyl pyrophosphate (IPP) to the more-reactive electrophile dimethylallyl pyrophosphate (DMAPP). This isomerization is a key step in the biosynthesis of isoprenoids through the mevalonate pathway and the MEP pathway.

In enzymology, bornyl diphosphate synthase (BPPS) (EC 5.5.1.8) is an enzyme that catalyzes the chemical reaction

Diphosphomevalonate decarboxylase (EC 4.1.1.33), most commonly referred to in scientific literature as mevalonate diphosphate decarboxylase, is an enzyme that catalyzes the chemical reaction
The enzyme sabinene-hydrate synthase (EC 4.2.3.11) catalyzes the chemical reaction
In enzymology, a dolichyldiphosphatase (EC 3.6.1.43) is an enzyme that catalyzes the chemical reaction

In enzymology, a nucleoside-diphosphatase (EC 3.6.1.6) is an enzyme that catalyzes the chemical reaction
In enzymology, an undecaprenyl-diphosphatase (EC 3.6.1.27) is an enzyme that catalyzes the chemical reaction
The enzyme prenyl-diphosphatase (EC 3.1.7.1) catalyzes the reaction
In enzymology, a farnesyltranstransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a rubber cis-polyprenylcistransferase (EC 2.5.1.20) is an enzyme that catalyzes the chemical reaction

In enzymology, an ATP phosphoribosyltransferase is an enzyme that catalyzes the chemical reaction









