A protein phosphatase is a phosphatase enzyme that removes a phosphate group from the phosphorylated amino acid residue of its substrate protein. Protein phosphorylation is one of the most common forms of reversible protein posttranslational modification (PTM), with up to 30% of all proteins being phosphorylated at any given time. Protein kinases (PKs) are the effectors of phosphorylation and catalyse the transfer of a γ-phosphate from ATP to specific amino acids on proteins. Several hundred PKs exist in mammals and are classified into distinct super-families. Proteins are phosphorylated predominantly on Ser, Thr and Tyr residues, which account for 79.3, 16.9 and 3.8% respectively of the phosphoproteome, at least in mammals. In contrast, protein phosphatases (PPs) are the primary effectors of dephosphorylation and can be grouped into three main classes based on sequence, structure and catalytic function. The largest class of PPs is the phosphoprotein phosphatase (PPP) family comprising PP1, PP2A, PP2B, PP4, PP5, PP6 and PP7, and the protein phosphatase Mg2+- or Mn2+-dependent (PPM) family, composed primarily of PP2C. The protein Tyr phosphatase (PTP) super-family forms the second group, and the aspartate-based protein phosphatases the third. The protein pseudophosphatases form part of the larger phosphatase family, and in most cases are thought to be catalytically inert, instead functioning as phosphate-binding proteins, integrators of signalling or subcellular traps. Examples of membrane-spanning protein phosphatases containing both active (phosphatase) and inactive (pseudophosphatase) domains linked in tandem are known, conceptually similar to the kinase and pseudokinase domain polypeptide structure of the JAK pseudokinases. A complete comparative analysis of human phosphatases and pseudophosphatases has been completed by Manning and colleagues, forming a companion piece to the ground-breaking analysis of the human kinome, which encodes the complete set of ~536 human protein kinases.

In biochemistry, a phosphatase is an enzyme that uses water to cleave a phosphoric acid monoester into a phosphate ion and an alcohol. Because a phosphatase enzyme catalyzes the hydrolysis of its substrate, it is a subcategory of hydrolases. Phosphatase enzymes are essential to many biological functions, because phosphorylation and dephosphorylation serve diverse roles in cellular regulation and signaling. Whereas phosphatases remove phosphate groups from molecules, kinases catalyze the transfer of phosphate groups to molecules from ATP. Together, kinases and phosphatases direct a form of post-translational modification that is essential to the cell's regulatory network.

The enzyme alkaline phosphatase has the physiological role of dephosphorylating compounds. The enzyme is found across a multitude of organisms, prokaryotes and eukaryotes alike, with the same general function but in different structural forms suitable to the environment they function in. Alkaline phosphatase is found in the periplasmic space of E. coli bacteria. This enzyme is heat stable and has its maximum activity at high pH. In humans, it is found in many forms depending on its origin within the body – it plays an integral role in metabolism within the liver and development within the skeleton. Due to its widespread prevalence in these areas, its concentration in the bloodstream is used by diagnosticians as a biomarker in helping determine diagnoses such as hepatitis or osteomalacia.

Nicotinamide adenine dinucleotide phosphate, abbreviated NADP+ or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). NADPH is the reduced form of NADP+, the oxidized form. NADP+ is used by all forms of cellular life.

In biochemistry, phosphorylases are enzymes that catalyze the addition of a phosphate group from an inorganic phosphate (phosphate+hydrogen) to an acceptor.
Cardiolipin is an important component of the inner mitochondrial membrane, where it constitutes about 20% of the total lipid composition. It can also be found in the membranes of most bacteria. The name "cardiolipin" is derived from the fact that it was first found in animal hearts. It was first isolated from the beef heart in the early 1940s by Mary C. Pangborn. In mammalian cells, but also in plant cells, cardiolipin (CL) is found almost exclusively in the inner mitochondrial membrane, where it is essential for the optimal function of numerous enzymes that are involved in mitochondrial energy metabolism.

The enzyme glucose 6-phosphatase (EC 3.1.3.9, G6Pase; systematic name D-glucose-6-phosphate phosphohydrolase) catalyzes the hydrolysis of glucose 6-phosphate, resulting in the creation of a phosphate group and free glucose:

Protein tyrosine phosphatases (EC 3.1.3.48, systematic name protein-tyrosine-phosphate phosphohydrolase) are a group of enzymes that remove phosphate groups from phosphorylated tyrosine residues on proteins:
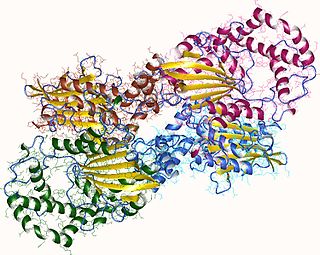
Acid phosphatase is an enzyme that frees attached phosphoryl groups from other molecules during digestion. It can be further classified as a phosphomonoesterase. It is stored in lysosomes and functions when these fuse with endosomes, which are acidified while they function; therefore, it has an acid pH optimum. This enzyme is present in many animal and plant species.
sn-Glycerol 3-phosphate is the organic ion with the formula HOCH2CH(OH)CH2OPO32-. It is one of three stereoisomers of the ester of dibasic phosphoric acid (HOPO32-) and glycerol. It is a component of glycerophospholipids. From a historical reason, it is also known as L-glycerol 3-phosphate, D-glycerol 1-phosphate, L-α-glycerophosphoric acid.

Phosphatidylglycerol is a glycerophospholipid found in pulmonary surfactant and in the plasma membrane where it directly activates lipid-gated ion channels.

The enzyme histidinol-phosphatase (EC 3.1.3.15) catalyzes the reaction

The enzyme phosphatidate phosphatase (PAP, EC 3.1.3.4) is a key regulatory enzyme in lipid metabolism, catalyzing the conversion of phosphatidate to diacylglycerol:
In enzymology, a CDP-diacylglycerol—glycerol-3-phosphate 3-phosphatidyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a [isocitrate dehydrogenase (NADP+)] kinase (EC 2.7.11.5) is an enzyme that catalyzes the chemical reaction:
In enzymology, a phosphatidylglycerol-membrane-oligosaccharide glycerophosphotransferase is an enzyme that catalyzes the chemical reaction

Phosphatidate cytidylyltransferase 1 is an enzyme that in humans is encoded by the CDS1 gene.
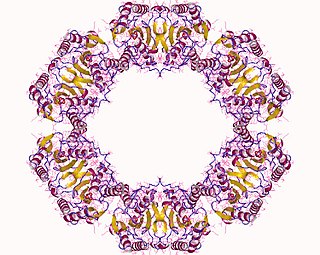
The enzyme protein serine/threonine phosphatase is a form of phosphoprotein phosphatase that acts upon phosphorylated serine/threonine residues:

Serine active site-containing protein 1, or Protein SERAC1 is a protein in humans that is encoded by the SERAC1 gene. The protein encoded by this gene is a phosphatidylglycerol remodeling protein found at the interface of mitochondria and endoplasmic reticula, where it mediates phospholipid exchange. The encoded protein plays a major role in mitochondrial function and intracellular cholesterol trafficking. Defects in this gene are a cause of 3-methylglutaconic aciduria with deafness, encephalopathy, and Leigh-like syndrome (MEGDEL). Two transcript variants, one protein-coding and the other non-protein coding, have been found for this gene.
(3-methyl-2-oxobutanoate dehydrogenase (2-methylpropanoyl-transferring))-phosphatase (EC 3.1.3.52, branched-chain oxo-acid dehydrogenase phosphatase, branched-chain 2-keto acid dehydrogenase phosphatase, branched-chain α-keto acid dehydrogenase phosphatase, BCKDH', [3-methyl-2-oxobutanoate dehydrogenase (lipoamide)]-phosphatase, [3-methyl-2-oxobutanoate dehydrogenase (lipoamide)]-phosphate phosphohydrolase) is an enzyme with systematic name (3-methyl-2-oxobutanoate dehydrogenase (2-methylpropanoyl-transferring))-phosphate phosphohydrolase. This enzyme catalyses the following chemical reaction













