Related Research Articles

Ribonuclease is a type of nuclease that catalyzes the degradation of RNA into smaller components. Ribonucleases can be divided into endoribonucleases and exoribonucleases, and comprise several sub-classes within the EC 2.7 and 3.1 classes of enzymes.

Ribonuclease H is a family of non-sequence-specific endonuclease enzymes that catalyze the cleavage of RNA in an RNA/DNA substrate via a hydrolytic mechanism. Members of the RNase H family can be found in nearly all organisms, from bacteria to archaea to eukaryotes.

Transcriptional modification or co-transcriptional modification is a set of biological processes common to most eukaryotic cells by which an RNA primary transcript is chemically altered following transcription from a gene to produce a mature, functional RNA molecule that can then leave the nucleus and perform any of a variety of different functions in the cell. There are many types of post-transcriptional modifications achieved through a diverse class of molecular mechanisms.
An esterase is a hydrolase enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis.
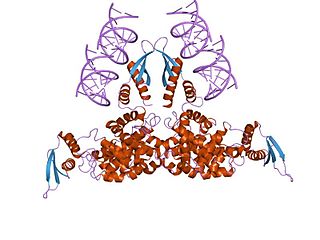
Ribonuclease III (RNase III or RNase C)(BRENDA 3.1.26.3) is a type of ribonuclease that recognizes dsRNA and cleaves it at specific targeted locations to transform them into mature RNAs. These enzymes are a group of endoribonucleases that are characterized by their ribonuclease domain, which is labelled the RNase III domain. They are ubiquitous compounds in the cell and play a major role in pathways such as RNA precursor synthesis, RNA Silencing, and the pnp autoregulatory mechanism.

Drosha is a Class 2 ribonuclease III enzyme that in humans is encoded by the DROSHA gene. It is the primary nuclease that executes the initiation step of miRNA processing in the nucleus. It works closely with DGCR8 and in correlation with Dicer. It has been found significant in clinical knowledge for cancer prognosis and HIV-1 replication.

Ribonuclease T1 (EC 3.1.27.3, guanyloribonuclease, Aspergillus oryzae ribonuclease, RNase N1, RNase N2, ribonuclease N3, ribonuclease U1, ribonuclease F1, ribonuclease Ch, ribonuclease PP1, ribonuclease SA, RNase F1, ribonuclease C2, binase, RNase Sa, guanyl-specific RNase, RNase G, RNase T1, ribonuclease guaninenucleotido-2'-transferase (cyclizing), ribonuclease N3, ribonuclease N1) is a fungal endonuclease that cleaves single-stranded RNA after guanine residues, i.e., on their 3' end; the most commonly studied form of this enzyme is the version found in the mold Aspergillus oryzae. Owing to its specificity for guanine, RNase T1 is often used to digest denatured RNA prior to sequencing. Similar to other ribonucleases such as barnase and RNase A, ribonuclease T1 has been popular for folding studies.
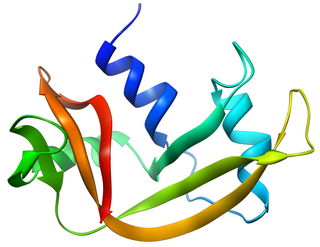
Pancreatic ribonuclease family is a superfamily of pyrimidine-specific endonucleases found in high quantity in the pancreas of certain mammals and of some reptiles.

Eosinophil-derived neurotoxin is an enzyme that in humans is encoded by the RNASE2 gene.

In enzymology, a polynucleotide adenylyltransferase is an enzyme that catalyzes the chemical reaction

Ribonuclease pancreatic is an enzyme that in humans is encoded by the RNASE1 gene.
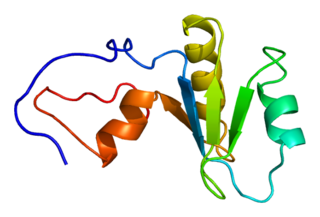
Poly(A)-specific ribonuclease (PARN), also known as polyadenylate-specific ribonuclease or deadenylating nuclease (DAN), is an enzyme that in humans is encoded by the PARN gene.

PAB-dependent poly(A)-specific ribonuclease subunit 2 is an enzyme that in humans is encoded by the PAN2 gene.

Ribonuclease H1 also known as RNase H1 is an enzyme that in humans is encoded by the RNASEH1 gene. The RNase H1 is a non-specific endonuclease and catalyzes the cleavage of RNA via a hydrolytic mechanism.
Ribonuclease T2 is an enzyme. It is a type of endoribonuclease. This enzyme catalyses the following chemical reaction
Ribonuclease IV is an enzyme. This enzyme catalyses the following chemical reaction
Ribonuclease is an enzyme. This enzyme catalyses the following chemical reaction
Ribonuclease E is a bacterial ribonuclease that participates in the processing of ribosomal RNA and the chemical degradation of bulk cellular RNA.
Ribonuclease U2 is an enzyme. This enzyme catalyses the following chemical reaction
Ribonuclease V is an enzyme. This enzyme catalyses the following chemical reaction
References
- ↑ Sideris DC, Fragoulis EG (April 1987). "Purification and characterization of a ribonuclease specific for poly(U) and poly(C) from the larvae of Ceratitis capitata". European Journal of Biochemistry. 164 (2): 309–15. doi:10.1111/j.1432-1033.1987.tb11059.x. PMID 3569265.