Related Research Articles
A protein phosphatase is a phosphatase enzyme that removes a phosphate group from the phosphorylated amino acid residue of its substrate protein. Protein phosphorylation is one of the most common forms of reversible protein posttranslational modification (PTM), with up to 30% of all proteins being phosphorylated at any given time. Protein kinases (PKs) are the effectors of phosphorylation and catalyse the transfer of a γ-phosphate from ATP to specific amino acids on proteins. Several hundred PKs exist in mammals and are classified into distinct super-families. Proteins are phosphorylated predominantly on Ser, Thr and Tyr residues, which account for 79.3, 16.9 and 3.8% respectively of the phosphoproteome, at least in mammals. In contrast, protein phosphatases (PPs) are the primary effectors of dephosphorylation and can be grouped into three main classes based on sequence, structure and catalytic function. The largest class of PPs is the phosphoprotein phosphatase (PPP) family comprising PP1, PP2A, PP2B, PP4, PP5, PP6 and PP7, and the protein phosphatase Mg2+- or Mn2+-dependent (PPM) family, composed primarily of PP2C. The protein Tyr phosphatase (PTP) super-family forms the second group, and the aspartate-based protein phosphatases the third. The protein pseudophosphatases form part of the larger phosphatase family, and in most cases are thought to be catalytically inert, instead functioning as phosphate-binding proteins, integrators of signalling or subcellular traps. Examples of membrane-spanning protein phosphatases containing both active (phosphatase) and inactive (pseudophosphatase) domains linked in tandem are known, conceptually similar to the kinase and pseudokinase domain polypeptide structure of the JAK pseudokinases. A complete comparative analysis of human phosphatases and pseudophosphatases has been completed by Manning and colleagues, forming a companion piece to the ground-breaking analysis of the human kinome, which encodes the complete set of ~536 human protein kinases.

In biochemistry, a phosphatase is an enzyme that uses water to cleave a phosphoric acid monoester into a phosphate ion and an alcohol. Because a phosphatase enzyme catalyzes the hydrolysis of its substrate, it is a subcategory of hydrolases. Phosphatase enzymes are essential to many biological functions, because phosphorylation and dephosphorylation serve diverse roles in cellular regulation and signaling. Whereas phosphatases remove phosphate groups from molecules, kinases catalyze the transfer of phosphate groups to molecules from ATP. Together, kinases and phosphatases direct a form of post-translational modification that is essential to the cell's regulatory network.

Inositol, or more precisely myo-inositol, is a carbocyclic sugar that is abundant in the brain and other mammalian tissues; it mediates cell signal transduction in response to a variety of hormones, neurotransmitters, and growth factors and participates in osmoregulation.

Phytic acid is a six-fold dihydrogenphosphate ester of inositol, also called inositol hexakisphosphate (IP6) or inositol polyphosphate. At physiological pH, the phosphates are partially ionized, resulting in the phytate anion.

Protein tyrosine phosphatases (EC 3.1.3.48, systematic name protein-tyrosine-phosphate phosphohydrolase) are a group of enzymes that remove phosphate groups from phosphorylated tyrosine residues on proteins:

A phytase is any type of phosphatase enzyme that catalyzes the hydrolysis of phytic acid – an indigestible, organic form of phosphorus that is found in many plant tissues, especially in grains and oil seeds – and releases a usable form of inorganic phosphorus. While phytases have been found to occur in animals, plants, fungi and bacteria, phytases have been most commonly detected and characterized from fungi.

Inositol oxygenase, also commonly referred to as myo-inositol oxygenase (MIOX), is a non-heme di-iron enzyme that oxidizes myo-inositol to glucuronic acid. The enzyme employs a unique four-electron transfer at its Fe(II)/Fe(III) coordination sites and the reaction proceeds through the direct binding of myo-inositol followed by attack of the iron center by diatomic oxygen. This enzyme is part of the only known pathway for the catabolism of inositol in humans and is expressed primarily in the kidneys. Recent medical research regarding MIOX has focused on understanding its role in metabolic and kidney diseases such as diabetes, obesity and acute kidney injury. Industrially-focused engineering efforts are centered on improving MIOX activity in order to produce glucaric acid in heterologous hosts.
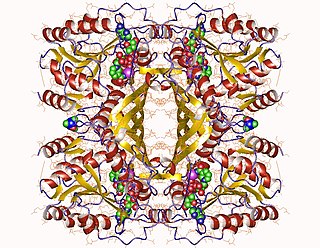
In enzymology, an inositol-3-phosphate synthase is an enzyme that catalyzes the chemical reaction
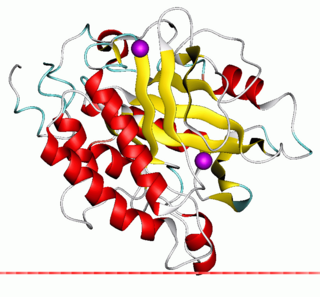
The enzyme phosphatidylinositol diacylglycerol-lyase catalyzes the following reaction:
The enzyme 3-phytase (EC 3.1.3.8) catalyzes the reaction
The enzyme 4-phytase (EC 3.1.3.26) catalyzes the following reaction:
The enzyme inositol-1,4-bisphosphate 1-phosphatase (EC 3.1.3.57) catalyzes the reaction

The enzyme Inositol phosphate-phosphatase is of the phosphodiesterase family of enzymes. It is involved in the phosphophatidylinositol signaling pathway, which affects a wide array of cell functions, including but not limited to, cell growth, apoptosis, secretion, and information processing. Inhibition of inositol monophosphatase may be key in the action of lithium in treating bipolar disorder, specifically manic depression.
The enzyme multiple inositol-polyphosphate phosphatase (EC 3.1.3.62) catalyzes the reaction

Inositol (1,4,5) trisphosphate 3-kinase (EC 2.7.1.127), abbreviated here as ITP3K, is an enzyme that facilitates a phospho-group transfer from adenosine triphosphate to 1D-myo-inositol 1,4,5-trisphosphate. This enzyme belongs to the family of transferases, specifically those transferring phosphorus-containing groups (phosphotransferases) with an alcohol group as acceptor. The systematic name of this enzyme class is ATP:1D-myo-inositol-1,4,5-trisphosphate 3-phosphotransferase. ITP3K catalyzes the transfer of the gamma-phosphate from ATP to the 3-position of inositol 1,4,5-trisphosphate to form inositol 1,3,4,5-tetrakisphosphate. ITP3K is highly specific for the 1,4,5-isomer of IP3, and it exclusively phosphorylates the 3-OH position, producing Ins(1,3,4,5)P4, also known as inositol tetrakisphosphate or IP4.
Dual-specificity phosphatase is a form of phosphatase that can act upon tyrosine or serine/threonine residues.
Inositol-hexakisphosphate kinase is an enzyme with systematic name ATP:1D-myo-inositol-hexakisphosphate 5-phosphotransferase. This enzyme catalyses the following chemical reaction
Phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase is an enzyme with systematic name 1-phosphatidyl-1D-myo-inositol-3,4,5-trisphosphate 5-phosphohydrolase, that has two isoforms: SHIP1 and SHIP2 (INPPL1).
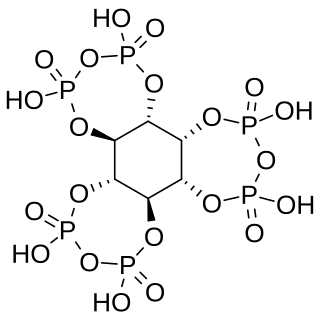
Myo-inositol trispyrophosphate (ITPP) is an inositol phosphate, a pyrophosphate, a drug candidate, and a putative performance-enhancing substance, which exerts its biological effects by increasing tissue oxygenation.

β-propeller phytases (BPPs) are a group of enzymes (i.e. protein superfamily) with a round beta-propeller structure. BPPs are phytases, which means that they are able to remove (hydrolyze) phosphate groups from phytic acid and its phytate salts. Hydrolysis happens stepwise and usually ends in myo-inositol triphosphate product which has three phosphate groups still bound to it. The actual substrate of BPPs is calcium phytate and in order to hydrolyze it, BPPs must have Ca2+ ions bound to themselves. BPPs are the most widely found phytase superfamily in the environment and they are thought to have a major role in phytate-phosphorus cycling in soil and water. As their alternative name alkaline phytase suggests, BPPs work best in basic (or neutral) environment. Their pH optima is 6–9, which is unique among the phytases.
References
- 1 2 Barrientos L, Scott JJ, Murthy PP (1994). "Specificity of hydrolysis of phytic acid by alkaline phytase from lily pollen". Plant Physiology. 106 (4): 1489–1495. doi:10.1104/pp.106.4.1489. PMC 159689 . PMID 7846160.
- 1 2 Puhl A, Greiner R, Selinger LB (2008). "A protein tyrosine phosphatase-like inositol polyphosphatase from Selenomonas ruminantium subsp. lactilytica has specificity for the 5-phosphate of myo-inositol hexakisphosphate". The International Journal of Biochemistry & Cell Biology. 40 (10): 2053–2064. doi:10.1016/j.biocel.2008.02.003. PMID 18358762.