
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process—from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials—to approved vaccine or drug typically takes more than a decade.

Nitazoxanide, sold under the brand name Alinia among others, is a broad-spectrum antiparasitic and broad-spectrum antiviral medication that is used in medicine for the treatment of various helminthic, protozoal, and viral infections. It is indicated for the treatment of infection by Cryptosporidium parvum and Giardia lamblia in immunocompetent individuals and has been repurposed for the treatment of influenza. Nitazoxanide has also been shown to have in vitro antiparasitic activity and clinical treatment efficacy for infections caused by other protozoa and helminths; evidence as of 2014 suggested that it possesses efficacy in treating a number of viral infections as well.
Leronlimab is a humanized monoclonal antibody targeted against the CCR5 receptor found on T lymphocytes of the human immune system. It is being investigated as a potential therapy in the treatment of COVID-19, triple negative breast cancer, and HIV infection. The United States Food and Drug Administration has designated PRO 140 for fast-track approval. In February 2008, the drug entered Phase 2 clinical trials and a phase 3 trial was begun in 2015. In February 2018, Cytodyn Inc reported that the primary endpoint had been achieved in the PRO 140 pivotal combination therapy trial in HIV infection. In 2020 CytoDyn submitted a fast-track biologics license application for treatment of CCR5-tropic HIV-1 Infection.

Losmapimod (GW856553X) is an investigational drug being developed by Fulcrum Therapeutics for the treatment of facioscapulohumeral muscular dystrophy (FSHD); a phase III clinical trial is pending approval. Losmapimod selectively inhibits enzymes p38α/β mitogen-activated protein kinases (MAPKs), which are modulators of DUX4 expression and mediators of inflammation.
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays.

Galidesivir is an antiviral drug, an adenosine analog. It was developed by BioCryst Pharmaceuticals with funding from NIAID, originally intended as a treatment for hepatitis C, but subsequently developed as a potential treatment for deadly filovirus infections such as Ebola virus disease and Marburg virus disease, as well as Zika virus. Currently, galidesivir is under phase 1 human trial in Brazil for coronavirus.

Drug repositioning is the repurposing of an approved drug for the treatment of a different disease or medical condition than that for which it was originally developed. This is one line of scientific research which is being pursued to develop safe and effective COVID-19 treatments. Other research directions include the development of a COVID-19 vaccine and convalescent plasma transfusion.

COVID-19 drug development is the research process to develop preventative therapeutic prescription drugs that would alleviate the severity of coronavirus disease 2019 (COVID-19). From early 2020 through 2021, several hundred drug companies, biotechnology firms, university research groups, and health organizations were developing therapeutic candidates for COVID-19 disease in various stages of preclinical or clinical research, with 419 potential COVID-19 drugs in clinical trials, as of April 2021.

The Solidarity trial for treatments is a multinational Phase III-IV clinical trial organized by the World Health Organization (WHO) and partners to compare four untested treatments for hospitalized people with severe COVID-19 illness. The trial was announced 18 March 2020, and as of 6 August 2021, 12,000 patients in 30 countries had been recruited to participate in the trial.

Molnupiravir, sold under the brand name Lagevrio, is an antiviral medication that inhibits the replication of certain RNA viruses. It is used to treat COVID‑19 in those infected by SARS-CoV-2. It is taken by mouth.

GS-441524 is a nucleoside analogue antiviral drug which was developed by Gilead Sciences. It is the main plasma metabolite of the antiviral prodrug remdesivir, and has a half-life of around 24 hours in human patients. Remdesivir and GS-441524 were both found to be effective in vitro against feline coronavirus strains responsible for feline infectious peritonitis (FIP), a lethal systemic disease affecting domestic cats. Remdesivir was never tested in cats, but GS-441524 has been found to be effective treatment for FIP.
The treatment and management of COVID-19 combines both supportive care, which includes treatment to relieve symptoms, fluid therapy, oxygen support as needed, and a growing list of approved medications. Highly effective vaccines have reduced mortality related to SARS-CoV-2; however, for those awaiting vaccination, as well as for the estimated millions of immunocompromised persons who are unlikely to respond robustly to vaccination, treatment remains important. Some people may experience persistent symptoms or disability after recovery from the infection, known as long COVID, but there is still limited information on the best management and rehabilitation for this condition.
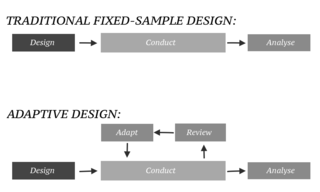
In an adaptive design of a clinical trial, the parameters and conduct of the trial for a candidate drug or vaccine may be changed based on an interim analysis. Adaptive design typically involves advanced statistics to interpret a clinical trial endpoint. This is in contrast to traditional single-arm clinical trials or randomized clinical trials (RCTs) that are static in their protocol and do not modify any parameters until the trial is completed. The adaptation process takes place at certain points in the trial, prescribed in the trial protocol. Importantly, this trial protocol is set before the trial begins with the adaptation schedule and processes specified. Adaptions may include modifications to: dosage, sample size, drug undergoing trial, patient selection criteria and/or "cocktail" mix. The PANDA provides not only a summary of different adaptive designs, but also comprehensive information on adaptive design planning, conduct, analysis and reporting.

Casirivimab/imdevimab, sold under the brand name REGEN‑COV among others, is a combination medicine used for the treatment and prevention of COVID‑19. It consists of two human monoclonal antibodies, casirivimab and imdevimab that must be mixed together and administered as an infusion or subcutaneous injection. The combination of two antibodies is intended to prevent mutational escape. It is also available as a co-formulated product. It was developed by the American biotechnology company Regeneron Pharmaceuticals.

BriLife, also known as IIBR-100, is a replication-competent recombinant VSV viral vectored COVID-19 vaccine candidate. It was developed by the Israel Institute for Biological Research (IIBR). The IIBR partnered with the US-based NRx Pharmaceuticals to complete clinical trials and commercialize the vaccine. A study conducted in hamsters suggested that one dose of the vaccine was safe and effective at protecting against COVID-19.

Nirmatrelvir is an antiviral medication developed by Pfizer which acts as an orally active 3C-like protease inhibitor. It is part of a nirmatrelvir/ritonavir combination used to treat COVID-19 and sold under the brand name Paxlovid.

COVID-19 vaccine clinical research uses clinical research to establish the characteristics of COVID-19 vaccines. These characteristics include efficacy, effectiveness, and safety. As of November 2022, 40 vaccines are authorized by at least one national regulatory authority for public use:

Nirmatrelvir/ritonavir, sold under the brand name Paxlovid, is a co-packaged medication used as a treatment for COVID‑19. It contains the antiviral medications nirmatrelvir and ritonavir and was developed by Pfizer. Nirmatrelvir inhibits SARS-CoV-2 main protease, while ritonavir is a strong CYP3A inhibitor, slowing down nirmatrelvir metabolism and therefore boosting its effect. It is taken by mouth.

Ensitrelvir, sold under the brand name Xocova is an antiviral medication used as a treatment for COVID-19. It was developed by Shionogi in partnership with Hokkaido University and acts as an orally active 3C-like protease inhibitor. It is taken by mouth.

The Platform Adaptive Trial of Novel Antivirals for Early Treatment of COVID-19 in the Community is a clinical trial in the United Kingdom to test the effectiveness of new antiviral drugs at the early stages of COVID-19 infections. The study aims to find out if antivirals can prevent the need for hospitalisation and help faster recovery for people aged over 50 and those at higher risk due to underlying health conditions. PANORAMIC is sponsored by the University of Oxford and funded by the National Institute for Health and Care Research (NIHR). The trial was launched in December 2021, and as of June 2022, over 25,000 people are enrolled as participants.













