 | |
 | |
| Names | |
|---|---|
| IUPAC name Chromium(III) acetate hydrate | |
| Other names chromic acetate, chromium triacetate, chromium(III) ethanoate | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.012.646 |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C12H36ClCr3O22 | |
| Molar mass | 723.84 g·mol−1 |
| Appearance | grayish-green to blueish-green solid |
| Density | 1.662 g/cm3 |
| Melting point | 1,152 [1] °C (2,106 °F; 1,425 K) |
| −5104.0·10−6 cm3/mol | |
| Structure | |
| octahedral | |
| Related compounds | |
Related compounds | Manganese(III) acetate Iron(III) acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
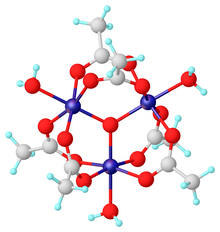
Chromium(III) acetate, commonly known as basic chromium acetate, [2] describes a family of salts where the cation has the formula [Cr3O(O2CCH3)6(OH2)3]+. The trichromium cation is encountered with a variety of anions, such as chloride and nitrate. Data in the table above are for the chloride hexahydrate, [Cr3O(O2CCH3)6(OH2)3]Cl(H2O)6.
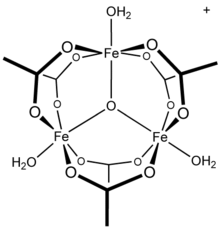
Salts of basic chromium acetate has long attracted interest because of its distinctive structure, which features octahedral Cr(III) centers, a triply bridging oxo ligand, six acetate ligands, and three aquo ligands. [2] The same structure is shared with basic iron acetate and basic manganese acetate. [2] [3] Little evidence exists for a simple chromium(III) acetate, i.e. lacking the oxo ligand. [4] Chromium(III) acetate is a blue/grey-green powder, which is soluble in water. It is still [3] prepared according to the original procedure from 1909. [5]